Discover Diabetes Connections | Type 1 Diabetes
Diabetes Connections | Type 1 Diabetes

Diabetes Connections | Type 1 Diabetes
Author: Stacey Simms
Subscribed: 434Played: 20,629Subscribe
Share
© Stacey Simms Media 2015-2026
Description
The T1D news show you've been waiting for! Long-time broadcaster, blogger and diabetes mom Stacey Simms interviews prominent advocates, authors and speakers. Stacey asks hard questions of healthcare companies and tech developers and brings on "everyday' people living with type 1. Great for parents of T1D kids, adults with type 1 and anyone who loves a person with diabetes.
774 Episodes
Reverse
It's In the News.. a look at the top headlines and stories in the diabetes community. This week's top stories: T1D in the Olympics & Superbowl, Trump RX goes live, Ozempic pill available soon, tech updates from Medtronic, Beta Bionics, Eversense 365 and more! Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Omnipod - Simplify Life All about Dexcom T1D Screening info All about VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Episode transcription with links: Welcome! I'm your host Stacey Simms and this is an In The News episode.. where we bringing you the top diabetes stories and headlines happening now. A reminder that you can find the sources and links and a transcript and more info for every story mentioned here in the show notes. Quick reminder: We are just over one week from our first Moms' Night Out event of the year. While the plans are all set – the speakers, the vendors, the raffles and the fun is ready to go, it's always amazing how many people hear of these event last minute. That's fine, they're welcome! But if you're thinking of attending a future event – registration is open for We're going to Nashville next March 6-7 and Detroit in September – no need to wait. And we've got Club 1921 events for health care professionals and patient leaders in 6 cities this year! All the info is over at diabetes-connetionss.com events/ Okay.. our top story this week: XX Gotta be a quick shout out to some incredible T1D athletes – we had TWO in the super bowl this past weekend – Chad Muma of the New England Patriots and Logan Brown of the Seattle Seahawks AND there are at least two athletes with type 1 competing at the Winter Olympics. Hannah Schmidt competes in ski cross for Canada – she was diagnosed with Type 1 diabetes at age 12 years old. Anna FarnSchadt Fernstäd a Czech skeleton racer diagnosed in 2022 after she'd already been to several Olympics. We wish them all the best! https://english.radio.cz/skeleton-racer-anna-fernstadtova-overcoming-adversity-headfirst-down-ice-8876699 XX The government website TrumpRx.gov is live.. the website does not sell prescription drugs. Instead, it allows people to look up their drugs and then navigate to buy them elsewhere, either from a major drug company or a pharmacy. The 43 drugs listed on the site have prices ranging from $3 to over $5,500. TrumpRx does include warnings that the site may not be the best option to save money on prescriptions. Each product page advises: "If you have insurance, check your co-pay first — it may be even lower." For now, the website says its prices are for people paying with their own money, rather than going through insurance. The only insulin listed right now is Lilly's insulin lispro – and it's the same price as you'd find through Illy's insulin value program. I looked up diabetes meds.. For example, if you have an insurance co-pay of $25 a month for Farxiga, a drug often used for diabetes, you would be paying $182 on TrumpRx. As you can imagine, though ,this is complicated and as with most of our healthcare system, it may be good in some cases and not much help in other. I'd suggest calling your local pharmacist or checking with your human resource dept. https://www.nytimes.com/2026/02/06/health/trumprx-prescription-drug-prices-consumers.html XX Novo Nordisk will launch some doses of its oral semaglutide for diabetes under the brand name Ozempic pill in the second quarter of this year. The company said the U.S. Food and Drug Administration has approved Ozempic tablets in three different doses. Novo says The new Ozempic name is intended to help patients and health care professionals more easily recognize the available treatment options for type 2 diabetes Semaglutide tablets have been available under the brand name Rybelsus Ruh BELL sis for diabetes since 2019 but with different dosing. The pill is also approved to reduce the risk of certain cardiovascular conditions in adults with type 2 diabetes who are at high risk for these events. The FDA had approved the new doses based on a bioequivalence study and the clinical trial data for Rybelsus, Novo said. https://www.reuters.com/business/healthcare-pharmaceuticals/novo-launch-ozempic-pill-diabetes-second-quarter-this-year-2026-02-04/ XX https://www.contemporarypediatrics.com/view/early-screening-for-type-1-diabetes-found-effective-in-children XX Possible new way to identify and track the progress of type 1 diabetes before clinical onset. A recent study published in Science Advances described the application of subcutaneous microporous scaffolds. These are inserted and have been shown to identify changes in cancer, multiple sclerosis, and T1D by capturing changes of immune cells over the course of a disease. This is a proof of concept study in mice.. so very early days. https://www.news-medical.net/news/20260204/Implantable-immune-scaffold-predicts-type-1-diabetes-weeks-before-symptoms.aspx XX A large global genetics study shows that many key drivers of Type 2 diabetes operate outside the bloodstream. In a major international project led in part by the University of Massachusetts Amherst and Helmholtz Munich in Germany, researchers linked hundreds of genes and proteins to the disease. The work, published in Nature Metabolism, points to a key challenge in diabetes research: the biology behind rising blood sugar does not play out the same way in every part of the body. It also shows why including people from many backgrounds matters, since genetic clues that stand out in one population may be faint or invisible in another. Huge study, 2.5 million people worldwide comparing patterns across seven tissues tied to diabetes and four global ancestry groups, then asked a simple question: what do you miss if you only measure blood? Across the seven tissues, the researchers found causal evidence pointing to 676 genes. Yet overlap with blood was limited: only 18% of genes with a causal effect in a primary diabetes tissue, such as the pancreas, showed a matching signal in blood. At the same time, 85% of genetic effects observed in diabetes-relevant tissues were completely absent from blood-based analyses. The findings lay out a roadmap for future research aimed at understanding the biological pathways underlying Type 2 diabetes and developing more effective treatments. https://scitechdaily.com/massive-global-study-rewrites-the-biology-of-type-2-diabetes/ XX Express Scripts settled the U.S. Federal Trade Commission's claims its insulin pricing practices violated antitrust and consumer protection laws, and agreed to changes aimed at lowering costs for patients, insurers and small pharmacies The settlement, first reported by Reuters, fits with that goal, and allows the FTC to pare down a case brought by the former Biden administration against Cigna's Express Scripts, UnitedHealth Group Inc's (UNH.N), Optum unit and CVS Health Corp's (CVS.N), CVS Caremark. The case against Optum and Caremark is ongoing. Pharmacy benefit managers, which set how drugs are covered by health insurance, have faced a decade of scrutiny from regulators and lawmakers over pricing practices. While the industry has already made reforms, the settlement gives the FTC power to enforce broader changes at Express Scripts. The 10-year agreement restricts Express Scripts' ability to engage in practices critics say contribute to high costs, like pocketing rebate payments from drugmakers based on the list price of drugs. The FTC estimates the agreement could save patients as much as $7 billion over a decade. https://www.reuters.com/world/cigna-settles-ftc-insulin-case-commits-overhauling-drug-pricing-2026-02-04/ XX Audio? Congress has passed bipartisan legislation to extend and strengthen the Special Diabetes Program (SDP), a cornerstone of Federal investment in type 1 diabetes (T1D) research. The President signed the legislation and it is now law. Extends the SDP through December 31, 2026, and increases funding from $160 million to $200 million annually. Strengthens overall funding for the National Institutes of Health (NIH) by $415 million. Increases diabetes research funding at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) by $10 million. Created by Congress and administered by the NIH, the SDP has contributed nearly $3.6 billion to T1D research and has played a role in nearly every major breakthrough in the field. A recent study conducted by Avalere Health shows that of the nearly 3.6 billion invested into the SDP by Congress since the establishment of the program, the Federal Government has realized $50 billion in healthcare savings through improved health outcomes from the use of SDP driven therapies and devices https://www.breakthrought1d.org/news-and-updates/congress-passes-bipartisan-extension-of-the-special-diabetes-program-securing-critical-t1d-research-funding/ XX Dexcom is rolling out what they're calling AI-enabled enhancements to Stelo, further transforming how users track and understand their glucose health. Expanded Smart Food Logging including a comprehensive nutrition database of more than 1M meals that provides a breakdown of calories, carbohydrates, protein, fat, dietary fibers, and more. More ways to meal track including text search, barcode scanning or taking a photo of the meal, creating a seamless and intuitive meal tracking solution. A redesigned Daily Insights feature which will introduce a new interface with more personalized recommendations. The newes
Two years ago the FDA pulled down just about every insulin calculator app. A lot of them just disappeared, rather than seek official approval, but one of them – created by a teenager with type 1 – is back. I'm talking with Drew and Mike Mendelow about the relaunch of T1D1, a free and ad-free insulin-dose calculator app. They share what it was like to navigate the FDA process, how they go international help, and what's next. This podcast is not intended as medical advice. If you have those kinds of questions, please contact your health care provider. Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Omnipod - Simplify Life All about Dexcom All about VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com
It's In the News.. a look at the top headlines and stories in the diabetes community. This week's top stories: UK looks at starting universal T1D screening, Dexcom's CEO mentions a new product, bariatric sugery vs GLP medications, FDA approves update to prescribing info for inhaled insulin, miscroplastic and diabetes link studied, and more! Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Omnipod - Simplify Life All about Dexcom T1D Screening info All about VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Episode transcription with links: (Stacey Track) Welcome! I'm your host Stacey Simms and this is an In The News episode.. where we bringing you the top diabetes stories and headlines happening now. We are less than one month from our first MNO of 2026. Please join us in Silver Spring MD Feb 20 and 21. It's going to be amazing. We're going to Nashville next March 6-7 and we're going to have a great event a Club 1921 we just added on Thursday March 5th for health care providers and patient leaders. All the info is over at diabetes-connetionss.com events/ Okay.. our top story this week: XX All UK children could be offered screening for type 1 diabetes using a simple finger-prick blood test, say researchers who have been running a large study. This is the ELSA study - Early Surveillance for Autoimmune diabetes, a first of its kind UK study. They tested blood samples from 17,931 children aged 3-13 for autoantibodies, markers of type 1 diabetes that can appear years before symptoms. Families of children found to have early-stage type 1 diabetes received tailored education and ongoing support to prepare for the eventual onset of type 1 diabetes symptoms and to ensure insulin therapy can begin promptly when needed, reducing the chances of needing emergency treatment. Those with one autoantibody also received ongoing support and monitoring. Some families were also offered teplizumab, the first ever immunotherapy for type 1 diabetes, which can delay the need for insulin by around three years in people with early-stage type 1 diabetes. The second phase has launched and will expand screening to all children in the UK aged 2-17 years, with a focus on younger children (2-3 years) and older teenagers (14-17 years). The research team aims to recruit 30,000 additional children across these new age groups. ELSA 2 will assess how screening can be scaled across the NHS and evaluate its cost-effectiveness. https://www.birmingham.ac.uk/news/2026/childhood-type-1-diabetes-screening-is-effective-and-could-prevent-thousands-of-emergency-diagnoses XX At the J.P. Morgan Healthcare Conference Dexcom CEO Jake Leach says they're going to launch a new product outside the US. I'll link up that interview, The full quote: "When you look at the outside the U.S., there are a lot of structures that are tiered. Patients have access to different types of products, so we've got a new one that we want to introduce that will add flexibility there. It's based on the G7 platform, just like Dexcom ONE+, but it has a unique experience that's tailored for a subset of users that, today, don't have access to Dexcom." Your guess is as good as mine, but sounds more like a pricing or ordering issue than a new bit of hardware or software. Dexcom will also bring Stelo to some international markets this year. And plans a new mobile app experience for the wearable biosensor meant for people who don't dose insulin. Leach also says G8 will be much smaller and with more capability. but is a few years away. https://www.drugdeliverybusiness.com/dexcom-ceo-jake-leach-2026-roadmap-jpm/ XX A new international consensus statement provides guidance for the use of diabetes technology during pregnancy for women with type 1 diabetes (T1D), type 2 diabetes (T2D), or gestational diabetes (GD). Organized by the diaTribe Foundation, the document was based on evidence where available, as well as opinion from an international group of experts in endocrinology, diabetes technology, and obstetrics & gynecology, among others. This is the first set of recommendations specifically addressing the use of diabetes technology in pregnancy – and we'll link it up. https://www.medscape.com/viewarticle/new-consensus-statement-addresses-diabetes-tech-pregnancy-2026a100020d XX Bariatric surgery beats GLP-1s for type 2 diabetes across income levels. This study was published this month, looking at nearly 300 patients are 4 medical centers. Success here is measured by lower blood glucose levels, higher weight loss (28% vs. 10%), less use of diabetes medications, remission of diabetes to the point of no longer needing to inject insulin, and reduced risk factors for cardiovascular disease. Bariatric surgery was better than medical therapy across all social backgrounds, they found, and not just in areas of higher deprivation. The ancillary study was smaller, and some of the participants randomized in earlier stages crossed over from medical to surgical treatment, and the reverse. The authors acknowledged and accounted for these limitations, along with the rapid development of more powerful obesity drugs not fully captured in the study. This was a long term study – more than 12 years – and by the end of the study more people were choosing GLP1 medications. One dividing line: If someone hopes to lose 100 pounds, that's more likely with surgery than with medications. "Ultimately, we need large, long-term, well-designed studies to clarify the best strategy for a given patient." https://www.statnews.com/2026/01/19/diabetes-study-bariatric-surgery-better-than-glp-1s/ XX Researchers at the University of California, Riverside have reported for the first time that a father's exposure to microplastics (MPs) can lead to metabolic problems in his children, including diabetes. This is a mouse study, but it looks at a previously unrecognized way in which environmental pollution may influence the health of future generations. MPs are extremely small plastic fragments, measuring less than 5 millimeters, that form as consumer products and industrial materials break down. Metabolic disorders describe a group of conditions that include elevated blood pressure, high blood sugar, and excess body fat, all of which raise the risk of heart disease and diabetes. The team found that female offspring of male mice exposed to MPs were far more prone to metabolic disorders than offspring of unexposed fathers, even though all offspring received the same high fat diet. The research team hopes the findings will guide future investigation into how MPs and even smaller nanoplastics affect human development. https://scitechdaily.com/microplastics-can-rewire-sperm-triggering-diabetes-in-the-next-generation/ XX The FDA has finalized four new recalls for certain lots of Abbott's FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors due to ongoing safety concerns. We told you about this in November when Abbott says some of its continuous glucose monitoring (CGM) sensors were providing incorrect low glucose warnings. Internal testing identified the issue—carbon building up in the sensors during the manufacturing process—and determined that approximately 3 million CGM sensors were affected. The sensors were distributed in the United States, Canada and several European countries. When Abbott shared that announcement, the FDA was still reviewing the situation. No recalls had yet been finalized. Now, however, the agency has announced four new Class I recalls. https://cardiovascularbusiness.com/topics/clinical/heart-health/fda-confirms-recalls-abbott-cgm-sensors-new-lawsuit-alleges-company-concealed-information XX Insulet brings back it's U.S. Pod recycling program, now making it available to all U.S. customers. The Pod recycling program, offered at no cost to customers, enables users to request a recycling kit online. This allows them to return their used Omnipods. Insulet then decontaminates the returned Pods before transporting them to a company specializing in recycling for electronics and medical products. Insulet began recycling pilot programs in Mass and California and are rolling it out nationwide. Insulet also has "Pod takeback" programs outside the U.S. in several international markets. These programs enable customers to request a takeback kit by contacting their local customer support team. https://www.drugdeliverybusiness.com/insulet-expands-us-pod-recycling-program/ XX Up next a new resource for a population at three times the risk for diabetes, but without a lot of access to health information. I The first diabetes information website primarily in ASL has launched. The site includes GIFs and videos on diabetes management and an ASL glossary of diabetes-related terms. This is from University of Utah Health – Called Deaf Diabetes Can Together. Deaf and hard of hearing people are at three times higher risk for diabetes, but access to health information in ASL is limited. https://healthcare.utah.edu/newsroom/news/2026/01/first-diabetes-information-website-asl-launches XX Novo Nordisk ended all work on cell therapies, including a Type 1 diabetes program, in October – and now has found a buyer. Aspect has acquired rights to the assets and giving Novo an option to reengage for later-stage development and commercialization. Novo is helping bankroll Aspect's development of the assets, investing in the company and providing research funding. The arrangement gives Novo a chance to p
Imagine getting your kids screened for T1D and agreeing to do it yourself, just to set a good example, and then your test is the one that comes back with type 1! That's exactly what happened to Chris Dunn. She was positive for all of the autoantibodies and has since been treated with Tzield, the medication shown to delay the onset. We're talking to her about all of that, what the treatment is actually like, how she's doing since and what her family thinks of the whole thing. This podcast is not intended as medical advice. If you have those kinds of questions, please contact your health care provider. Join us for an All Things Camp webinar TONIGHT 1/20 8pm ET Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Omnipod - Simplify Life All about Dexcom All about VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com
It's In the News... the top diabetes stories and headlines happening now! Top stories this week include: new islet encapsulation trial, FDA agrees to review Tzield for babies and approves the MiniMed Go, Civica releases it's version of Lantus, Eversense launches with a pump partner, scholarship for college students with diabetes and more! Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life All about Dexcom Screen it Like You Mean It All about VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Episode transcript: Welcome! This is an In the News episode.. we're doing things a little differently this year, but we are still bringing you the top diabetes stories and headlines happening now. We are rotating these shorter episodes with our longer, interview episodes.. but all on Tuesdays now. And that's why you'll hear ads on the in the news episodes. My usual disclaimer – we believe in the products advertised here but no one tells me what stories to run or what to say – the commercials are separate from the news. I know most podcasters don't do disclaimers anymore, but I come from traditional broadcasting and it's still important to me. Okay.. our top story this week: XX Positive results for a phase 1 clinical trial of encapsulated islets in people with type 1 from Encellin. This first-in-human trial is assessing (1) safety and adverse events, (2) cell survival within the device, and (3) fibrosis, or scarring, around the implants. As planned, ENCRT devices were removed from the initial five participants after 4 months of implantation. Analysis of the removed devices show: minimal to no fibrosis (formation of scar tissue around the device); robust formation of blood vessels around the device; viable islets inside the device. These results indicate that the device has the potential to host islet cells while maintaining sufficient oxygen and nutrient flow for the cells to work effectively. Breakthrough T1D's Role: Encellin's technology was originally developed within the Lab of Dr. Tejal Desai (University of California San Francisco) with funding from Breakthrough T1D, which was crucial in the formation of Encellin as a company and attracting subsequent venture capital. https://breakthrought1d.ca/cell-therapy-trial-encellins-device-delivers-promising-early-results/ XX The US Food and Drug Administration has accepted Tzield for priority review to expand the current age indication from eight years and above, to as young as one year old and above to delay the onset of stage 3 type 1 diabetes (T1D) in patients diagnosed with stage 2 T1D. The target action date for the FDA decision is April 29, 2026. "This priority review emphasizes the urgent need for innovative therapies like Tzield which has the potential to prevent the natural progression of T1D by delaying the loss of endogenous insulin production. This might be particularly significant in this young population, as it is well documented that the autoimmune attack that drives this disease in many cases, begins, early in life," said Christopher Corsico, Global Head of Development at Sanofi. "If approved, Tzield could represent an important advance for delaying the onset of stage 3 type 1 diabetes in early childhood, which would benefit patients and caregivers alike." Interim data for the PETITE-T1D phase 4 study was presented at the 51st Annual Conference of the International Society for Pediatric and Adolescent Diabetes and simultaneously published in Diabetologia. Priority review is given to regulatory applications seeking approval for therapies that have the potential to provide significant improvements in the treatment, diagnosis, or prevention of serious conditions. The safety and efficacy of Tzield in the PETITE-T1D population has not been approved by any regulatory authority. https://www.globenewswire.com/news-release/2026/01/05/3212420/0/en/Press-Release-Sanofi-s-Tzield-accepted-for-priority-review-in-the-US-for-young-children-with-stage-2-type-1-diabetes.html XX Sanofi said the U.S. Food and Drug Administration accepted a priority review to potentially expand the current age range for its Tzield type-1 diabetes drug to include children as young as one year old. The French pharmaceutical company said Monday that the FDA's review could amend the lower end of the range for the drug, which is currently approved for children as young as eight years old. Tzield would be the first disease-modifying therapy that works to delay stage three type-1 diabetes for children aged one and older—who currently have stage two of the disease, according to Sanofi. The review comes after positive data from a Phase 4 study, and the anticipated action date for the FDA decision is April 29, Sanofi said. https://finance.yahoo.com/news/sanofi-says-fda-agrees-review-064000083.html Separately, The European Commission approved Sanofi's Teizeild (teplizumab) to delay the onset of stage 3 type 1 diabetes (T1D) in adult and paediatric patients eight years of age and older with stage 2 disease, the company announced Monday. A positive opinion had been issued by the EMA's Committee for Medicinal Products for Human Use in November. https://firstwordpharma.com/story/7057996 XX The FDA clears the MiniMed Go app for multiple daily injections (MDI). MiniMed Go, a smart MDI system, integrates the InPen smart insulin pen with the Instinct sensor made by Abbott. Medtronic and Abbott entered a collaboration in August 2024 to pair insulin delivery and continuous glucose monitoring (CGM) technologies. Medtronic (soon to be a standalone company called MiniMed) launched the MiniMed 780G automated insulin delivery system with the Instinct sensor made by Abbott late last year. Now, the technology can pair with Medtronic's other insulin delivery offering, the InPen smart insulin pen. MiniMed Go received clearance for individuals with insulin-requiring type 1 and type 2 diabetes aged seven years and older. It also received the nod for children aged 2-6 under the supervision of an adult caregiver. Compatibility for Medtronic's own Simplera sensor with MiniMed Go remains under FDA review. Medtronic expects to launch MiniMed Go in the U.S. in the coming spring. https://www.drugdeliverybusiness.com/medtronic-fda-clearance-minimed-go-inpen-instinct/ XX A new UNC-led study, published in the Journal of Diabetes and Its Complications, is the first to highlight diabetes distress that people over the age of 65 can encounter when managing T1D, including the unique stressors in this age group. While the researchers found that markedly elevated levels of diabetes distress were less prevalent among older adults than in younger age groups, understanding the sources of that distress – and the people most likely to experience it – will help health care and public health experts improve treatment strategies for this unique population. Around 36% of older adults surveyed in the study reported elevated levels of diabetes distress warranting further assessment and treatment. Older adults who identified as women, had higher hemoglobin A1C levels and had been to the emergency room in the past year were most likely to experience elevated levels of diabetes distress. The most common stressors included financial worries, T1D management difficulties and worries about complications. An unexpected finding was that people who had been diagnosed with T1D at earlier ages or had lived with the condition the longest had lower levels of diabetes distress. "Our group hopes to continue exploring the specific factors and experiences that contribute to diabetes distress in this population, and this study has highlighted areas for potential next steps. This work also closely aligns with ongoing projects our research team is conducting, including a clinical trial, ChargeUp." https://sph.unc.edu/sph-news/new-study-highlights-stressors-of-living-with-type-1-diabetes-as-an-older-adult/ XX The FDA announced its plan to stop subjecting non-medical grade wearable devices to FDA regulations in an effort to clarify the agency's approach on AI and digital health. The guidance builds on the agency's existing policy classifying low-risk wellness tools, such as fitness apps and activity trackers encouraging exercise, as non-medical devices exempt from tight regulation, provided they don't associate claims related to disease diagnosis or treatment. When asked about the accuracy of these general wellness devices Makary told Fox News, "If they're not making claims that they are medical grade, let's let the market decide. Let's let doctors choose from a competitive marketplace which ones they recommend for their patients." I'm watching this closely, curious to see which will be the first diabetes related product through the gate. General Wellness: Policy for Low Risk Devices U.S. Food and Drug Association January 6, 2026https://www.fda.gov/regulatory-information/search-fda-guidance-documents/general-wellness-policy-low-risk-devices https://www.pharmexec.com/view/fda-limits-regulations-non-medical-grade-wearable-fitness-devices XX Senseonics announced today that it began the launch of its Eversense 365 sensor with the Sequel Med Tech twiist pump. This is the first pump pairing with the implantable one-year CGM. The system features what they're calling iiSure technolo
One of the sure-thing top stories of 2026 will be GLP1s, but will we see more studies and even approval for treating type 1 with these medications? We're talking about Ozempic, Mounjaro and the next versions like Retatrutide - that are just around the corner. I'm talking to Dr. Cecelia Lo Wong, a diabetologist at the University of Colorado whose been on the front lines of this conversation for years, including serving on FDA advisory committees. This is a wide ranging interview - we also talk about the growing needs of older adults with type 1 diabetes, how kidney and cardiovascular risk guidelines are evolving, and why managing diabetes in the hospital can still be such a challenge. This podcast is not intended as medical advice. If you have those kinds of questions, please contact your health care provider. This week's Community Commercial is from Lisa Katzenberger, the author of "It Belongs to the World," a children's book version of the story of Frederick Banting and the discovery of insulin. Learn more about this book here Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life All about Dexcom All about VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com
It's In the News.. a look at the top headlines and stories in the diabetes community. This week we're also making predictions for diabetes news in 2026! This week's top stories: statins and type 2, big results from Lilly's newest weight loss drug, MiniMed IPO, Tandem app update, and more! Predicitons include thouhts around: continuous ketone monitoring, noninvasive gucose moniotring, inhalable insulin for kids, GLP1 backlash, A1 slop in diabetes mom groups and more! Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life All about Dexcom All about VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com
This week on Diabetes Connections, a conversation about what really matters in diabetes. We're talking about the top stories of 2025, the hype that didn't happen, some trends for 2026, what community can accomplish, and what LeBron James has to do with all of this. We also get personal – because I'm being interviewed by the wonderful Neil Greathouse, host of Your Best T1D life, and so much more. This episode contains a replay of Your Best T1D Year. Check out Neil's great podcast! This podcast is not intended as medical advice. If you have those kinds of questions, please contact your health care provider. Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life All about Dexcom All about VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com
Making the case for a better at home A1C test. Orange Biomed is developing a compact, one-drop, at-home A1C testing device they say could make frequent A1C checks easier and more accessible than ever. They're passionate about closing the gap for people who struggle to get to clinics regularly… and the research they share is compelling: four A1C tests a year can lead to a nearly 4% reduction in A1C levels. We'll talk about why more frequent A1C monitoring matters—even in the era of continuous glucose monitoring—how their new device works, and what early clinical trial results look like. This podcast is not intended as medical advice. If you have those kinds of questions, please contact your health care provider. More about Orange BioMed here Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life Learn about Dexcom Check out VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Episode transcript: Stacey Simms 00:05 Today on diabetes connections, making the case for a better at home A1C test. Orange biomed is developing a compact, one drop device that they say could make frequent A1C checks easier and more accessible. They're sharing research that four A1C tests a year can lead to a nearly 4% reduction in A1C levels, but they say a lot of people can't get to the clinic that much. We'll talk about why this matters, even in the era of CGM, how the device works and what the early clinical trial results look like. This podcast is not intended as medical advice. If you have those kinds of questions, please contact your healthcare provider. Welcome to a bonus episode of diabetes connections. I hope your December is going well and that you know somehow you're able to take some time for yourself in the middle of all the holiday rushing around this time of year can be magical and stressful and exhausting and wonderful, and you know, all the things. And it's the same thing over here, super busy getting all this stuff done before the end of the year. Love it. But, you know, getting podcast episodes out, writing all the things we write and planning for next year, as they say, We're staying booked and busy. But quick behind the scenes here to better explain this episode, I taped this interview way back over the summer during the ADA Scientific Sessions conference. I had some technical problems. I actually thought I lost this interview. There were two interviews that seemed to have gone missing. We're going to air the other one very soon. But thankfully, I do have backups upon backups. So all the info that you're going to hear today is still relevant. This product, a small A1C test, is still in development. The only dated bit is about their follow up event that took place in August. Orange Biomed was launched in 2021 in South Korea, with its US headquarters in Providence, Rhode Island. Its founders are two Duke University alums, and they're my guests, CEO Yeaseul Park and Co-President Unghyeon Ko, We are also joined by Janice Dru-Bennett. She is a senior advisor at the company. Now, English is not the first language of two of these three speakers. This is a good time to remind you that most podcasting platforms have pretty good transcription services these days, especially Apple, I think they have a fantastic real time transcription service for podcasts that has been impressive to me in how they translate diabetes language. They're getting better at it. But I am also going to put a transcript of the interview in the show notes, which I don't normally do because the podcast services have gotten so good at it, but I think it could be helpful for at least a few of you out there. Okay, here is my conversation from the floor of ADA from the team at Orange biomed. Yeaseul Park, Janice Drew Bennett and Dr Ko, Welcome to diabetes connections. I can't say live from ADA, because we're taping this to air later, but you're all there. Thank you so much for joining Yeaseul Park 03:08 me. Yes, thank you. We're 03:10 excited to be here. Oh Stacey Simms 03:11 my goodness. Can I ask first, how is the trip? I mean, yes, let me ask you. You guys came a long way. Yeaseul Park 03:17 Yeah, it was 13 hours from Korea. But it's I'm so excited, because this event is really one of the times, and this is actually our third time attending ADA. Stacey Simms 03:31 That's great. And we have so many questions for you, but Janice, let me ask you, you're there as everybody's setting up at the kind of beginnings of the show. What is it like right now for people who aren't familiar with ADA, Janice Dru-Bennett 03:42 yes, you can hear the hammers in the background, although, but not on this podcast, but there's a lot of noise and people walking by. We're just setting up this the day before the exhibit hall opens and Dr Cole will be presenting at the Innovation Hub tomorrow, which is where we're sitting right now, with tables of innovators will be showcasing their diabetes innovations, and Stacey Simms 04:04 there's a lot to get to. Dr Koh, I know you're presenting, but yes, let me ask you, like, what why? I know you said it's your third year, but why is orange biomed at ADA, what is your goal Yeaseul Park 04:16 for us? ADA, is for a learning experience. As well as a platform to share. We come to see how all those around the world are fighting against diabetes, whether through clinical research, digital tools or technologies or community programs. At the same time, you're so proud to hear what orange biomat is building anytime, and eight months exhausting. That makes diabetes monitoring not accessible, not so many. And this year is especially exciting because Dr ko our co founder of orange buying at the group of speaking at ADA brand new program the innovation Hall. Stacey Simms 04:58 That's awesome. So Dr Koh, tell me. Little bit about this, the Innovation Hub is pretty cool, but what are you going to be talking about? Unghyeon Ko 05:05 Yeah, actually, I'm talking about the engineering part. I mean our technology, so our orange biomed, we are trying to solve a simple but a serious problem about the A1C accessibility. So to increase the A1C accessibility. So we are, we are developing at home device to measure the A1C level. So I'm, I'm talking about how difficult to increase the accessibility of A1C, but our technology is handled that difficult problem. So we now he's so agreed. So I'm going to introduce our technology and emphasize the importance of the A1C measurement at home. Stacey Simms 05:49 Yeah, so A1C, it's interesting. My son was diagnosed at two, and in the pediatric world, you know, they'll just prick a finger generally and have that A1C right away. But my husband lives with type two, and he gets his labs drawn. And then it takes forever. So tell me a little bit before we go further about what you're hoping to do and making this easier for the patient, Speaker 1 06:10 the frequent monitoring of A1C is so important to prevent the diabetic complications. So the money, so if you there is some so I can say that there is a research that if you measure the A 1d the four times a year, the People's A1C level is decreased like 3.8% but if you measure the A 1d at one per year, Then the A1C level is increased 1.5% so the frequent A1C monitoring is so important to prevent the diabetes complications. But problem is A1C measurement is only available at clinical site at this moment, so most of the A1C monitoring is done by the clinical side. So that's why people are difficult to monitoring A1C, because they have to visit the clinics forever. So is so like four times, or even eight times visit the clinics or hospital is quite difficult, especially in the people living in the far area from the hospital. So that's why the home A1C test is required. So I think that's why the accessibility of the A1C is one of the important things in managing the diabetes complications. Stacey Simms 07:39 Dr Koh, is there evidence that, I mean more frequent A1C testing, I think would give many people peace of mind, perhaps. But is there evidence that it really does help in your health? Speaker 1 07:51 Oh, yes, it is actually like from there is the research, like the famous research about the A1C level, like the research name this t and this research proved that the A1C is the one of the strongest predictor of diabetes complication. So A1C is completely related with the risk of diabetes complication. So like keeping A1C on the 7% dramatically lower the risk of diabetes complications. And also, there is another research in UK, the UK PDS study, and that study said they are A1C. Lowering A1C by just 1% can reduce overall mortality by 15% and microvascular complication by 37% so the roaring A1C is the goal of the treatment of the Yeaseul Park 08:47 diabetes. So Stacey Simms 08:48 when I think of at home diabetes tests, blood tests, seem like they're they're really sensitive, right? You have to be very careful with things like that, although we do, we did finger sticks at home for years and years. Are there challenges with at home A1C testing that that people like me could mess up, Yeaseul Park 09:06 sure actually when I was doing pandemic outside system? So it's a new Yeaseul Park 09:19 box of mustard with five or six needles inside, and we need to collect
What if your glucose graph became a tangible piece of art? Something you could pring out and put on your water bottle or the back of your laptop. I've seen this in person and it makes a big impact on people. This week I'm talking to Krista Shenaman about making this type of art, her journey with type 2 – and it's been a journey, she took a "record breaking" 28 day walk after her diagnosis.. – why she thinks its helpful to look at data in a new way and more. Full disclosure: We recorded this interview way back in 2024! Technical issues and thought it was lost, but we found it. This podcast is not intended as medical advice. If you have those kinds of questions, please contact your health care provider. Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life Learn about Dexcom Check out VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Reach out with questions or comments: info@diabetes-connections.
It's been a big month for announcements from Dexcom! What does that mean for you? From the commercial launch of the 15 day sensor and a smart basal feature to the announced phase out of the G6 and more, I'm talking with Jessica Castle, vice president of Global Medical Affairs at Dexcom. We're covering all of this news and she's answering your questions. More about the Dexcom 15 day here More about Dexcom G6 phase out here This podcast is not intended as medical advice. If you have those kinds of questions, please contact your health care provider. Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life Learn about Dexcom Check out VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Reach out with questions or comments: info@diabetes-connections.
We're looking at some major policy issues happening in Washington, and what you can really do to effect change. George Huntley is the CEO of DPAC, the Diabetes Patient Advocacy Coalition. We've got a lot to cover: Medicare changes like competitive bidding that could dramatically limit access to CGMs and insulin pumps for seniors, the changing landscape around GLP 1 meds, and we talk about patient advocacy wins. I know some of you are cynical, but it can work. If you've ever thought your voice doesn't matter, this conversation may change your mind. This podcast is not intended as medical advice. If you have those kinds of questions, please contact your health care provider. Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life Learn about Dexcom Check out VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Reach out with questions or comments: info@diabetes-connections. Keywords Diabetes, D-PAC, Medicare, GLP-1 medications, patient advocacy, healthcare access, insulin pumps, CGMs, diabetes technology, legislative reform AI info below: Summary In this conversation, George Huntley, CEO of the Diabetes Patient Advocacy Coalition (D-PAC), discusses the critical role of advocacy in improving diabetes care and access to technology. He highlights the challenges faced by patients, particularly regarding Medicare coverage for insulin pumps and continuous glucose monitors (CGMs), and the implications of recent legislative changes. The discussion also covers the potential of GLP-1 medications in diabetes management and the importance of patient stories in advocacy efforts. Takeaways D-PAC focuses on affordable and equitable access to diabetes care. Advocacy is crucial for influencing healthcare policies. Competitive bidding for diabetes technology could limit access for seniors. Patient stories are essential in legislative advocacy. GLP-1 medications show promise in reshaping diabetes treatment. Economic factors play a significant role in healthcare access. The aging population of type 1 diabetes patients requires urgent attention. Collaboration among advocacy groups is vital for success. Healthcare costs are driven more by major medical expenses than by drug prices. Continued advocacy is necessary to protect patient access to care. Chapters 00:00 Introduction to Diabetes Advocacy 03:01 The Role of D-PAC in Diabetes Care 05:53 Challenges in Medicare Coverage for Diabetes Technology 09:11 The Impact of Competitive Bidding on Seniors 11:55 Advocacy Efforts and Legislative Challenges 14:57 The Future of GLP-1 Medications 17:56 Economic Implications of Diabetes Management 21:01 The Importance of Patient Advocacy 23:59 Healthcare Costs and Insurance Dynamics 26:56 The Need for Continued Advocacy 29:54 Conclusion and Call to Action
It's In the News.. a look at the top headlines and stories in the diabetes community. This week's top stories: big FDA recall around Freestyle Libre (see more below to find out if you're affected), Dexcom launches their 15.5 day sensor, Omnipod announces enhancements, Tandem tests a fully closed loop (with high fat, high carb meals) and lots more! Find out how to submit your Community Commercial Find out more about Moms' Night Out Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life Learn about Dexcom Check out VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Twitter Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Reach out with questions or comments: info@diabetes-connections.com Episode transcription with links: Hello and welcome to Diabetes Connections In the News! I'm Stacey Simms and every other Friday I bring you a short episode with the top diabetes stories and headlines happening now. Our top story this week: XX Certain glucose monitors from Abbott Diabetes Care are providing users with incorrect glucose readings, an error that has been linked with the deaths of at least seven people and more than 700 serious injuries worldwide, according to an alert from the US Food and Drug Administration. Incorrect glucose readings can lead to improper treatment. Abbott warned that about 3 million FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors are affected, but no other Libre products. Patients can visit FreeStyleCheck.com to see if their sensors are affected and to get a replacement for free. The FDA has also published specific information about the affected products in its alert. The agency considers this to be a "potentially high-risk issue" and will continue to update its website as information becomes available. "Patients should verify if their sensors are impacted and immediately discontinue use and dispose of the affected sensor(s)," the FDA said. https://www.cnn.com/2025/12/02/health/abbott-diabetes-glucose-monitors https://www.freestylecheck.com/us-en/home.html XX Omnipod 5 is getting some enhancements.. and Omnipod 6 is announced. The FDA cleared updates including a lower, 100 mg/dL target glucose option and what they call a more seamless automated experience. "This is the most significant algorithm advancement to our Omnipod 5 System since its launch in 2022," said Eric Benjamin, Insulet EVP and COO. Insulet said the new 100 mg/dL target glucose expands Omnipod 5's customization range. It now features six settings between 100 mg/dL and 150 mg/dL in 10 mg/dL increments. The company said this flexibility allows healthcare providers to tailor insulin delivery more precisely. It supports individuals seeking tighter glucose management or aiming to meet specific glucose goals. Omnipod 5's latest upgrades also help users stay in "Automated Mode" with fewer interruptions, even during prolonged high glucose events. Insulet plans to launch the updates to the algorithm in the first half of 2026. The company announced plans for an Omnipod 6 – without a lot of detail - at the company's Investor Day event in November. They also talked about a new, fully closed-loop pump for the type 2 diabetes population. https://www.drugdeliverybusiness.com/insulet-fda-clearance-omnipod-5-algorithm-enhancements/ XX Dexcom, the global leader in glucose biosensing, announced today that the Dexcom G7 15 Day Continuous Glucose Monitoring (CGM) System will launch in the United States on Dec. 1, making it the longest-lasting CGM system with 15.5 days of wear. Dexcom G7 15 Day will first be available through durable medical equipment (DME) providers on Dec. 1 with full retail launch in the coming weeks. Dexcom G7 15 Day will also be covered for Medicare beneficiaries. Dexcom G7 15 Day's industry-leading wear-time will provide fewer sensor changes, less disruption and more time for people with diabetes to benefit from life-changing CGM technology. New with Dexcom G7 15 Day: Longest lasting CGM system with 15.5 days of wear. Best-in-class accuracy1 with an overall MARD of 8.0%. Easier glucose management with fewer monthly sensor changes and reduced monthly waste. This follows yesterday's announcement – the FDA has cleared Dexcom Smart Basal, the first and only CGM-integrated basal insulin dosing optimizer designed for adults 18 and older with Type 2 diabetes using long-acting insulin. Dexcom Smart Basal will use Dexcom G7 15 Day sensor data and logged doses to calculate personalized daily recommendations to guide users towards a more effective long-acting insulin dose, as directed by their healthcare provider. At launch, Dexcom G7 15 Day will connect with the iLet Bionic Pancreas and Omnipod® 5§§. We are working closely with Tandem and look forward to extending the launch to their customers shortly as they finalize integration. For specific information on pump compatibility and availability with the Dexcom G7 15 Day system, visit Dexcom.com/connectedpumps https://investors.dexcom.com/news/news-details/2025/Dexcom-G7-15-Day-Continuous-Glucose-Monitoring-System-to-Launch-on-Dec--1-in-the-United-States/default.aspx XX A small study of ten adults with type 1 diabetes tested Tandem's new fully closed-loop "Freedom" insulin system — and the participants put it through a real-world stress test. For 72 hours in a hotel setting, they ate heavy carb-and-fat meals, skipped all meal announcements, and didn't give any mealtime insulin boluses. The system handled almost everything automatically. Researchers said the device stayed in closed-loop mode 97% of the time and there were no incidents of diabetic ketoacidosis or severe hypoglycemia reported. While using the Freedom system, participants spent a median 61% of the day in the glucose target range — slightly higher than the 56% achieved with their usual pump at home. But the biggest improvement came overnight: time in range jumped to 96% with the closed-loop system compared to just under 70% during their home-pump week. With almost zero time spent below 70 mg/dL, researchers concluded that the fully automated Tandem system was both safe and effective even with unannounced, high-impact meals — hinting at a future of diabetes management that demands less effort from users. XX Novo Nordisk reported promising mid-stage results for its experimental drug amycretin (AM-ee-creht-in) in diabetes patients on Tuesday. Amycretin, targets both GLP-1 and amylin hormones. In this study, it helped patients with type 2 diabetes lose up to 14.5% of their body weight over 36 weeks with weekly injections, far outperforming a placebo. The oral version delivered weight loss of up to 10.1%. Rival Eli Lilly is surging ahead with its own amylin-based drug, eloralintide, which is advancing to late-stage testing after helping patients shed as much as 20% of their weight in a mid-stage trial. https://www.cnbc.com/2025/11/25/novos-next-gen-obesity-drug-shows-positive-results-heads-to-late-stage-testing.html XX The U.S. Medicare health plan said on Tuesday that newly negotiated prices for 15 of its costliest drugs will save 36% on those medications compared with recent annual spending, or about $8.5 billion in net covered prescription costs. The prices go into effect in 2027, including a monthly price of $274 for Novo Nordisk's popular GLP-1 drug semaglutide, sold as Wegovy for weight loss and Ozempic for diabetes. medicare's recent net price for Ozempic, opens new tab was $428 a month, according to an analysis published in the Journal of Managed Care and Specialty Pharmacy. Medicare put the drug's list price, before confidential rebates and discounts, at $959 a month. Based on such nondiscounted list prices, Medicare said savings on the 15 drugs ranged from 38% to 85%. The annual price negotiations were established under President Joe Biden's signature Inflation Reduction Act (IRA) of 2022. Previously, Medicare was barred by law from negotiating with drugmakers. https://www.reuters.com/business/healthcare-pharmaceuticals/us-negotiated-medicare-prices-15-more-drugs-test-cost-savings-promise-2025-11-25/ XX LifeScan announced its Chapter 11 bankruptcy reorganization plan received U.S. Bankruptcy Court approval. LifeScan said it's positioned to emerge from its financial restructuring process by the end of the year. The CEO says, "This balance sheet restructuring provides a stronger foundation for LifeScan to support our base business, advance new growth strategies, and commence our journey to become one of the most comprehensive players in the glucose management space." https://www.drugdeliverybusiness.com/glucose-monitor-lifescan-emerge-from-bankruptcy/ XX An artificial intelligence (AI)-led Diabetes Prevention Program (DPP) was as effective as a traditional human-led program in achieving recommended goals for weight loss, A1c reduction, and physical activity, according to a randomized trial of adults with prediabetes and overweight or obesity. One example of a push notification: "Looks like you're at the grocery store, Rita! Want a quick list of high-fiber snacks or smart swaps to stay on track this week?" The app also provided location- and goal-based education, with gamification elements to promote engagement. Approximately one third of participants in both the AI and human-led groups achieved the primary outcome (31.7% and 31.9%, respectively). Results were consistent across sensitivity analyses and individual components of the composite endpoint. "As more AI-based programs emerge, head-to-head comparisons
We've got a research update on two of the topics you've told me you want to hear more about. First, research into Preventing type 1 – with a therapy that hasn't been in the headlines.. and second, inhaled insulin for kids. We're talking to Dr. Michael Haller, a peds endo who is at the forefront of these studies. We'll be talking about something called ATG – which looks really good in very low dose trials – and about the latest studies around inhaled insulin for kids – which is in front of the FDA right now. ATG studies and info here Afrezza for kids info here This podcast is not intended as medical advice. If you have those kinds of questions, please contact your health care provider. Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life Learn about Dexcom Check out VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Reach out with questions or comments: info@diabetes-connections.
With lots of family time coming up this week for many of us, it's a great time to talk about screening for type 1. While this might seem to be a real downer of a Thanksgiving conversation, screening is now considered standard of care for people with a family history of T1D. My guests want to get the word out about that – and they've both walked the walk. Adam Schefter is ESPN Senior NFL Insider – his wife lives with type 1.. and Dr. Shara Bialo is a pediatric endo who lives with type 1. This podcast is not intended as medical advice. If you have those kinds of questions, please contact your health care provider. More information about screening here Our previous episodes about TZIELD here Listen to our Thanksgiving themed episodes here Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life Learn about Dexcom Check out VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Reach out with questions or comments: info@diabetes-connections.
The Diabetes Sprots project says it's an organization built to inspire. What can we all learn about elite athletes with type 1 – the people running marathons and doing Iron Man competitions. And with the right support and education, how far can those athletes go? We're talking about the Olympics and more with DSP founder Casey Boren and volunteer Lauren Adams, both of whom live with type 1 (and both of whom had done a ten mile run before we started taping). Learn more about Diabetes Sports Project here This podcast is not intended as medical advice. If you have those kinds of questions, please contact your health care provider. Announcing Community Commericals! Learn how to get your message on the show here. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life Learn about Dexcom Check out VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Reach out with questions or comments: info@diabetes-connections.
It's In the News.. a look at the top headlines and stories in the diabetes community. This week's top stories: It's World Diabetes Day and we have a LOT of news to get to! Daily oral insulin tested to prevent T1D, mothers and sons and a T1D link, stem cell updates, Tandem Android news, Omnipod's workplace campaign and more! Find out how to submit your Community Commercial Find out more about Moms' Night Out Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life Learn about Dexcom Check out VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Twitter Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Reach out with questions or comments: info@diabetes-connections.com Episode transcription with links: Hello and welcome to Diabetes Connections In the News! I'm Stacey Simms and every other Friday I bring you a short episode with the top diabetes stories and headlines happening now. It's world diabetes day! It is marked every year on 14 November, the birthday of Sir Frederick Banting, who co-discovered insulin along with Charles Best in 1922. WDD was created in 1991 by International Diabetes Federation (IDF) and the World Health Organization and became an official United Nations Day in 2006 with the passage of United Nations Resolution 61/225. There will be a ton of stuff in your feeds today and that's great! I'm going to keep this to a pretty normal in the news episode.. although I do have my own World Diabetes Day announcement – I want YOUR community commercials. You could have an ad for your event or your blog or your project right here! There's a post on the website explaining it all and I'll come back at the end of the episode and tell you more. XX The Primary Oral Insulin Trial (POInT) is the first large-scale clinical trial to test whether giving at-risk children daily oral insulin could prevent or delay type 1 diabetes (T1D). Conducted by researchers from Helmholtz Munich and the Technical University of Munich across five European countries, the study enrolled more than 1,000 children with a genetic risk for T1D. Results published in The Lancet show that while oral insulin did not prevent the development of islet autoantibodies—an early sign of diabetes—it was safe and well tolerated. Importantly, researchers found that some children who received oral insulin developed diabetes more slowly than those given a placebo, suggesting potential protective effects in certain genetic subgroups. Further analysis revealed that the response to treatment depended on the child's insulin gene variant. Children with genetic versions that raise diabetes risk appeared to benefit, showing delayed onset of the disease, while those without the risk variant did not. These findings point toward a future of personalized prevention, where genetic screening could help identify which children might benefit most from oral insulin. Researchers will continue following the participants until age 12 to assess long-term effects. The study marks a major milestone in decades of diabetes prevention research, highlighting both the promise and complexity of developing tailored, early interventions against type 1 diabetes. XX Joint US-Chinese research looking at generating new beta cells from stomach cells. Upon turning on the "genetic switch," the human stomach cells were converted to insulin-secreting cells within the mice and resembled pancreatic beta cells with respect to gene and protein expression. Encouragingly, when those experiments were done with diabetic mice, insulin secreted from the transformed human cells helped control blood sugar levels and ameliorated diabetes. The scientists hope that a similar approach can be taken to convert cells from a patient's own stomach into insulin-secreting cells directly within the body. Importantly, additional studies are needed to address if this approach is safe and effective to be used in patients. https://www.technologynetworks.com/cell-science/news/human-stomach-cells-tweaked-to-make-insulin-406694 XX A new study in Nature Metabolism may help explain why children born to mothers with type 1 diabetes are less likely to develop the disease early in life compared to those whose fathers or siblings have it. Researchers looked at nearly 2,000 mothers and their children and found that kids whose moms have type 1 diabetes show changes in their DNA that may actually help protect them. These aren't genetic mutations, but epigenetic changes — chemical tags that turn certain genes on or off. The study found these changes in genes tied to the immune system and type 1 diabetes risk, suggesting that a mother's condition during pregnancy can shape her child's immune response in a protective way. Scientists identified more than 500 areas of DNA where these changes occurred, many in regions that control how the body's immune system works. Most of the changes appeared to calm down the kind of overactive immune response that leads to type 1 diabetes. Researchers even created a "methylation score" to help measure this protective effect. They say the next step is to confirm these results in more diverse groups and figure out exactly how these DNA changes help prevent early diabetes. https://www.news-medical.net/news/20251110/Maternal-type-1-diabetes-may-protect-children-from-developing-the-disease.aspx XX A new study from Karolinska Institutet and Stockholm University reveals that sons born to mothers with type 1 diabetes may develop early vascular dysfunction—independently of metabolic health. The finding may help shape future strategies to prevent cardiovascular disease early in life. Children of women with type 1 diabetes are known to be at increased risk of developing cardiovascular diseases. This new study, published in Cell Reports Medicine, is the first to show that the risk is linked to early dysfunction in blood vessel cells in sons, even before any metabolic issues arise. The team is now investigating the long-term effects of maternal diabetes, with a particular focus on why sons seem to be affected earlier than daughters. https://medicalxpress.com/news/2025-11-sons-mothers-diabetes-early-vascular.html XX A new study presented at Kidney Week 2025 has shown that the drug finn-uh-near-own a nonsteroidal mineralocorticoid-receptor antagonist, significantly reduced albuminuria—a key marker of kidney damage—in people with type 1 diabetes (T1D) and chronic kidney disease (CKD). This is the first major breakthrough for this population in more than 30 years. Researchers found that patients taking finerenone saw a 25% average reduction in albuminuria compared to placebo, an improvement that suggests a lower long-term risk for dialysis or kidney transplant. The phase 3 FINE-ONE trial involved 242 adults with T1D and CKD, and results showed benefits as early as three months. The drug was generally well tolerated, with side effects similar to those seen in patients with type 2 diabetes, though mild hyperkalemia (high potassium levels) was slightly more common. Experts say the findings could change the way doctors treat kidney complications in type 1 diabetes, an area that hasn't seen new therapies since the early 1990s. Currently, treatment options rely on blood pressure and blood sugar management, along with renin-angiotensin system (RAS) inhibitors. Finerenone, which is already approved for type 2 diabetes-related CKD, targets overactivation of a receptor that drives kidney damage. Based on these results, Bayer plans to seek FDA approval in 2026 for use in people with T1D and CKD. Researchers and clinicians alike are calling the study "groundbreaking," noting that it opens the door to future research on how finerenone might not just slow kidney decline—but possibly prevent it altogether. https://www.medscape.com/viewarticle/finerenone-offers-hope-kidney-disease-type-1-diabetes-2025a1000uzi?form=login XX This week, Tandem Diabetes Care (Nasdaq:TNDM) announced a major milestone for its Mobi miniature durable insulin pump system. San Diego-based Tandem revealed that it received FDA approval for the Android version of its Mobi mobile app. Clearance brings Mobi — which the company describes as the world's smallest, durable automated insulin delivery system — to more users. The pump, which pairs with Tandem's Control-IQ+ algorithm, previously worked with iOS software. Tandem — one of the largest diabetes tech companies in the world — expects to begin a limited rollout next month, followed by full commercial availability in early 2026. This marks the latest milestone for the company, which continues to expand its offerings and widen its reach within the diabetes patient population. We had a great interview with Tandem on our previous episode, but as I said at the time, it was coming before their earnings call. So here's an update: The company plans to submit the tubeless mobi to the fda before the end of this year.. possible approval and shipping date is hoped for by middle of 2026. Trials for their fully closed loop next-generation algorithm which we tlkaed abou ton the show should be launched in 2026 The Sigi patch pump will be developed and launched as a next-generation version of the Mobi Great job by Dr. David ? Ahn – he posted on IG after getting a message from tandem CEO John Sheridan? 1. First, the Tandem X3 *is* still absolutely in development, contrary to my speculation In yesterday's video. As many of you appropriately pointed out, there is definitely a market for a 300 unit pump, a pump with a screen, and a pump that does not re
We've got an update from Tandem Diabetes. We're talking about Libre 3 plus integration, Lyumjev approval, Mobi tubeless, extended wear infusion sets and a lot more with VP of Product Management Marisa Fienup. She's also answering your questions about tubing, alerts, and shares what's next. This podcast is not intended as medical advice. If you have those kinds of questions, please contact your health care provider. Announcing Community Commericals! Learn how to get your message on the show here. Information on RocketAP and other closed loop research Tandem earnings call info here Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life Learn about Dexcom Check out VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Reach out with questions or comments: info@diabetes-connections.
Taking a photo of your food and getting an accurate carb count seemed like a pipe dream just a few years ago, but this week's guest says it's here. Snaq wants to help you get nutritional info, and then see how that food actually affects blood glucose, thanks to integrations with CGMs, insulin pumps, and activity trackers. Snaq founder Aurelian Briner explains how his wife's type 1 diagnosis inspired the company, how it all works (and who owns the data), and what's next. Learn more about Snaq here Clinical Trial with Snaq here This podcast is not intended as medical advice. If you have those kinds of questions, please contact your health care provider. Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life Learn about Dexcom Check out VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Reach out with questions or comments: info@diabetes-connections.
What can those of us who will never climb the highest mountains, or take on extreme outdoor challenges – learn from those who do? Patrick Mertes and Michael Shelver have hiked and biked – and fallen – on incredible expeditions, while living with type 1. They'll share what they've learned, how those lessons apply to everyday life with type 1, what their family's think and what's next. Plus, Patrick talks about why diabetes camps remain such a cornerstone of confidence and connection, and how parents can help their kids face challenges that truly build independence. This is the keynote address from Moms' Night Out Minneapolis. Make sure to join us in 2026 for this amazing event! More info: at the Moms' Night Out main page This podcast is not intended as medical advice. If you have those kinds of questions, please contact your health care provider. Learn more about Project 50 in 50 Learn more about studies and research at Thrivable here Please visit our Sponsors & Partners - they help make the show possible! Learn more about Gvoke Glucagon Gvoke HypoPen® (glucagon injection): Glucagon Injection For Very Low Blood Sugar (gvokeglucagon.com) Omnipod - Simplify Life Learn about Dexcom Check out VIVI Cap to protect your insulin from extreme temperatures The best way to keep up with Stacey and the show is by signing up for our weekly newsletter: Sign up for our newsletter here Here's where to find us: Facebook (Group) Facebook (Page) Instagram Check out Stacey's books! Learn more about everything at our home page www.diabetes-connections.com Reach out with questions or comments: info@diabetes-connections.




















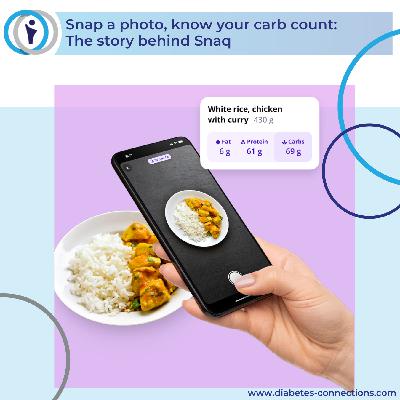




I ♥️ this episode! I've listened to it multiple times...the ideas give me so much hope!
I will checkout the diabetes summit online in oct 2017.
great POD cast. listen to it weekly