Fat Metabolism Holds the Key to Why We Need Sleep
Description
STORY AT-A-GLANCE
Sleep is not just downtime but a built-in survival mechanism that protects your brain from toxic byproducts created when mitochondria leak electrons
New research shows that the more electrons your cells fail to use, the greater the buildup of reactive oxygen species, which directly triggers your need for sleep
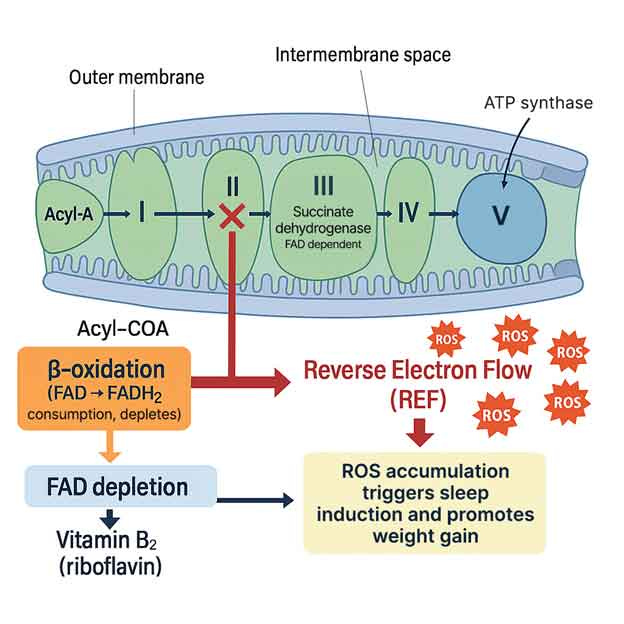
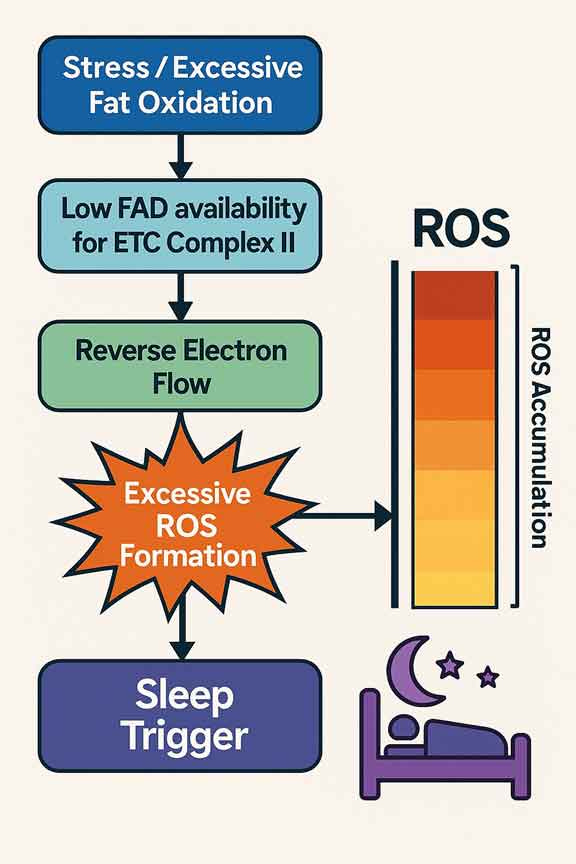
Excessive fat burning under stress makes this problem worse by clogging energy pathways, depleting cofactors, and pushing your body into deep fatigue
Serotonin levels rise when fatty acids flood your system, creating another pathway that drives drowsiness and heavy sleep pressure
Supporting your mitochondria with healthy carbs, avoiding extreme cardio, and limiting harmful fats found in seed oils lowers electron leaks, reduces sleep demand, and helps you feel more energized

Sleep shapes every part of your health, yet for decades its true purpose remained unclear. You have likely heard that it restores memory, balances hormones, or strengthens immunity — but none of those explanations fully answered why life cannot continue without it.
What scientists now recognize is that sleep is hardwired into your biology as a survival mechanism. The process of creating energy in your cells isn’t perfectly clean. Each day, your mitochondria — the power plants inside every cell — leak electrons and generate toxic byproducts. These molecules are so harmful that your brain forces you into sleep, shutting down activity so your body can repair the damage before it spirals out of control.
Understanding sleep in this way reframes it as a metabolic safeguard, not wasted time. It explains why you feel heavy fatigue after stressful days or long bouts of endurance exercise — the fuel mix in your body has shifted in ways that clog your energy system and accelerate cellular stress. At the same time, it shows why people with healthier metabolisms often get by with far less sleep: their mitochondria run cleaner, leak fewer electrons, and create less damage to repair.
This perspective opens the door to a deeper question: what exactly happens inside your cells that builds the pressure to sleep, and how do those changes play out across your brain and body? A new study, in which researchers mapped the mitochondrial shifts that create the pressure to sleep, set out to find the answer.
Mitochondria Signal When It’s Time to Sleep
A paper published in Nature examined fruit flies to uncover what triggers the brain’s need for sleep.1 Researchers wanted to understand why prolonged wakefulness produces such a strong drive to rest, and they focused on the activity inside specific neurons that regulate sleep. They discovered that when flies were deprived of sleep, their sleep-control neurons dramatically shifted how their mitochondria functioned.
The research focused on specialized sleep-control neurons — The researchers looked at a small group of neurons known as dorsal fan-shaped body neurons (dFBNs), which act like switches that decide when the fly sleeps or stays awake. These cells showed major changes after sleep loss, while other neurons in the brain did not. This specificity helped pinpoint exactly where sleep pressure originates, making it easier to track how mitochondria play a direct role.
Mitochondria change shape and function after sleep loss — The researchers found that in these neurons, genes responsible for making energy surged after sleep deprivation. Mitochondria fragmented into smaller units, and more contact points formed with the endoplasmic reticulum — a structure in the cell that helps with repair and lipid processing. These changes pointed to stress on the mitochondria and a greater need to manage toxic byproducts.
Sleep pressure was directly linked to electron overflow — The study showed that when mitochondria handled more electrons than they could safely process, they leaked extra electrons, creating reactive oxygen species (ROS), toxic molecules that damage cells. When mitochondria were modified to reduce this electron leak, flies needed less sleep. On the other hand, when electron leakage was increased, flies fell asleep faster and stayed asleep longer.
Sleep need was manipulated by changing mitochondrial activity — By forcing mitochondria to use up more electrons through special proteins, researchers reduced the flies’ sleep time. When they blocked the normal use of electrons and forced a backup of the system, the flies slept more. This demonstrated that sleep is tightly tied to the balance of energy demand and toxic byproduct cleanup inside neurons.
Mitochondria are the true regulators of sleep — According to the researchers, “Sleep, like aging, may be an inescapable consequence of aerobic metabolism.” The mitochondria act like sensors, detecting when the balance between fuel burned and energy used tips too far. When electron leaks rise, mitochondria send signals that trigger your brain’s sleep circuitry. This ensures your body slows down, lowers activity, and allows repair systems to catch up before permanent damage occurs.
In essence, sleep restores cellular balance — It’s more than rest — it’s an emergency response system to protect your brain from energy stress. By forcing downtime, your body prevents runaway damage from ROS and restores healthy mitochondrial function. This means that the efficiency of your metabolism directly shapes how much sleep you require.