83. RFJC 13 – ARDS Series – DEXA-ARDS
Update: 2024-08-27
Description
In the penultimate episode in our ARDS Rapid Fire Journal Club Summer Series we are talking about the DEXA-ARDS trial (published in Lancet Respiratory Medicine in 2020). This trial evaluated the impact of dexamethasone in the treatment of ARDS.
<figure class="aligncenter size-large">
 </figure>
</figure><figure class="aligncenter size-large">
 </figure>
</figure>
Article and Reference
Today we’re discussing the DEXA-ARDS trial published in Lancet Respiratory Medicine in 2020. This trial evaluated the impact of dexamethasone on mortality and duration of mechanical ventilation for patients with ARDS.
Infographic
<figure class="aligncenter size-full is-resized">
 </figure>
</figure>
Article Notes
- DEXA-ARDS; Lancet Respiratory Medicine, 2020
- DOI:10.1016/S2213-2600(19)30417-5
- Link: https://doi.org/10.1016/s2213-2600(19)30417-5
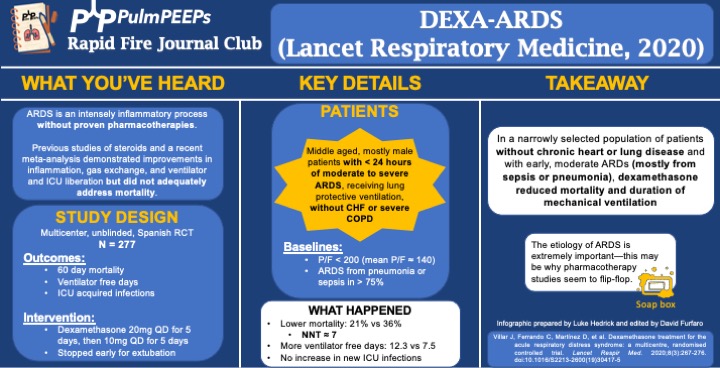
- Background: ARDS is an intense inflammatory process without proven, specific pharmacotherapies. Previous work and a recent meta-analysis demonstrated improvements in inflammation, gas exchange, and ventilator and ICU liberation but did not adequately address mortality.
- Study Design (design, primary outcome, participants, etc)
- Design: investigator-initiated, multicenter, unblinded, randomized controlled trial in 17 academic ICUs in Spain, conducted from 3/2013 to 12/2018
- Primary Outcome
- VFD at 28d
- Secondary:
- 60d mortality
- Actual duration of ventilation in ICU survivors
- ICU acquired infections
- Participants
- Inclusion ARDS with P/F < 200 for < 24hr on LTVV
- Exclusion:
- Already receiving steroids or immunosuppression
- CHF
- Severe COPD
- DNR
- Summary: Middle aged, mostly male patients with < 24hr of moderate to severe ARDS receiving LPV without chronic heart or lung disease
- Like many ARDS trials, just over 3/4 of patients’ ARDS was caused by PNA or sepsis. Mean P/F was ~140
- Intervention/Limitations
- N = 277, stratified by center and then randomized
- Intervention: dexamethasone 20mg qd for 5d followed by 10mg qd for 5d
- Stopped early for extubation before day 10
- First dose given no more than 30 hours after P/F < 200
- Control: no placebo, just SOC
- All patients received LTVV
- Outcomes/Safety
- Power: with N = 314 (actual N = 277), 80% power to detect 2 additional VFD and 15% mortality reduction
- As an aside, this seems to be a theme in ICU trials: massively ambitious proposed benefits during power calculations and then under-enrolling for that power calculation ultimately resulting with a point estimate that favors the intervention but is not statistically significant.
- Efficacy:
- 60d mortality: 21% vs 36%, P = 0.0047
- NNT of just < 7!
- VFD at 28d: 12.3 vs 7.5, P < 0.0001
- Actual duration of ventilation in ICU survivors: 14.2d vs 19.5d (P = 0.0009)
- 60d mortality: 21% vs 36%, P = 0.0047
- Safety:
- Hyperglycemia: 76% vs 70%, P = 0.33
- Always interesting in steroid trials when no change in glucose control is seen. This isn’t the most EBM thing I’ll ever say, but frankly I disregard this and assume steroids will cause hyperglycemia regardless of the trial results.
- ICU acquired infections: 24% vs 25%, P = 0.75
- Hyperglycemia: 76% vs 70%, P = 0.33
- Power: with N = 314 (actual N = 277), 80% power to detect 2 additional VFD and 15% mortality reduction
- Takeaway
- In a narrowly selected population of patients without chronic heart or severe lung disease<span style="font-weight: 4
Comments
Top Podcasts
The Best New Comedy Podcast Right Now – June 2024The Best News Podcast Right Now – June 2024The Best New Business Podcast Right Now – June 2024The Best New Sports Podcast Right Now – June 2024The Best New True Crime Podcast Right Now – June 2024The Best New Joe Rogan Experience Podcast Right Now – June 20The Best New Dan Bongino Show Podcast Right Now – June 20The Best New Mark Levin Podcast – June 2024
In Channel





