Regulatory science and the development of microbiome biomarkers, with Dr. Céline Druart PhD
Description
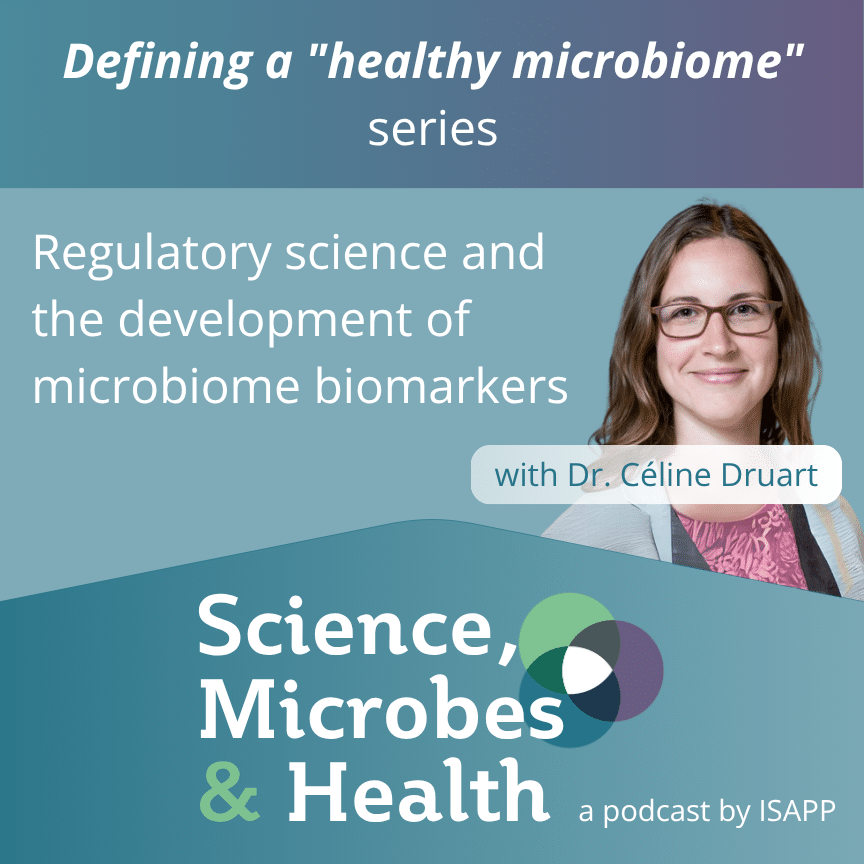
This episode features Dr. Céline Druart PhD of Pharmabiotic Research Institute (PRI) speaking about the role of regulatory science in the development of microbiome biomarkers. First, PRI’s Communication and Membership Manager Camille Bello briefly describes the work of the PRI in supporting the development of therapeutic and diagnostic products derived from microbiome science. Regulation is important to protect consumers and reward innovation, and the development of biomarkers that predict response to treatment, for example, can help move toward personalized medicine. Dr. Druart notes many potential microbiome-based biomarkers have emerged but none have been successfully validated to date. Regulation always follows innovation, and regulatory science is important because it helps regulatory frameworks evolve. A recent Delphi consensus paper co-authored by Dr. Druart acknowledges that biomarker development is a complex process and that a particular challenge is the lack of validation of analytical methods for measuring the microbiome. However, it’s important to remember that techniques can continue to improve even after they’ve been validated. Dr. Druart argues that biomarker validation needs public-private collaborations to design and execute the large clinical studies that are necessary.
Episode abbreviations and links:
- Recent paper co-authored by Dr. Druart: State of the art and the future of microbiome-based biomarkers: a multidisciplinary Delphi consensus
- PRI website: https://pharmabiotic.org/
- PRI LinkedIn page: https://www.linkedin.com/company/pharmabiotic-research-institute-pri
- PRI conference, February 3–5, 2026: https://www.pharmabioticsevent.com/
About Dr. Céline Druart PhD:
Céline Druart obtained her PhD in Biomedical and Pharmaceutical Sciences from UCLouvain (Belgium) in 2014. Following a 3-year project in Patrice Cani’s research group focused on developing the potential beneficial effects of a human gut commensal Akkermansia muciniphila, she worked for 3 years at A-Mansia Biotech (now known as The Akkermansia Company), responsible for clinical programs, regulatory affairs and IP dossiers, as the Scientific and Business Project Manager. Céline joined the PRI in July 2021 as Microbiome Project Manager, managing the Regulatory Science activities of the Association, coordinating Task Group work, and supporting PRI Industry Members in their development planning. She became the PRI’s Executive Director in January 2024.
Sign up for our monthly newsletter
Follow us on LinkedIn, Bluesky, X, Facebook, Instagram, Threads