Discover Aging-US
Aging-US

Aging-US
Author: Aging-US Podcast
Subscribed: 6Played: 213Subscribe
Share
© All rights reserved
Description
Aging-US is dedicated to advancing our understanding of the biological mechanisms that drive aging and the development of age-related diseases. Our mission is to serve as a platform for high-quality research that uncovers the cellular, molecular, and systemic processes underlying aging, and translates these insights into strategies to extend healthspan and delay the onset of chronic disease.
Read about the Aging (Aging-US) Scientific Integrity Process: https://aging-us.com/scientific-integrity
Read about the Aging (Aging-US) Scientific Integrity Process: https://aging-us.com/scientific-integrity
538 Episodes
Reverse
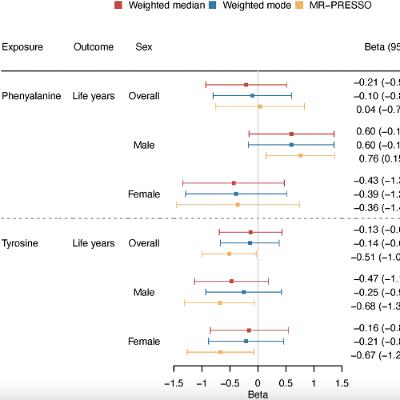
BUFFALO, NY — November 13, 2025 — A new #research paper was #published in Volume 17, Issue 10 of Aging-US on October 3, 2025, titled “The role of phenylalanine and tyrosine in longevity: a cohort and Mendelian randomization study.”
In this study led by Jie V. Zhao, Yitang Sun, Junmeng Zhang, and Kaixiong Ye from the University of Hong Kong and the University of Georgia, researchers investigated whether two amino acids, phenylalanine and tyrosine, affect how long people live (lifespan). The results suggest that higher levels of tyrosine are linked to shorter life expectancy in men, pointing to potential sex-specific approaches to promoting longevity.
Phenylalanine and tyrosine are amino acids involved in metabolism and brain function. Both are found in protein-rich foods and dietary supplements, but their long-term effects on aging are not well understood. Tyrosine, in particular, is a building block of neurotransmitters such as dopamine, which regulate mood and cognitive function, making it a molecule of interest in aging research.
The study analyzed data from more than 270,000 individuals in the UK Biobank. Using both observational and genetic methods, the researchers examined the associations between blood levels of phenylalanine and tyrosine with overall mortality and predicted lifespan. Although both amino acids were initially linked to higher mortality risk, only tyrosine showed a consistent and potentially causal association with reduced life expectancy in men. Genetic analyses estimated that elevated tyrosine levels could shorten men’s lifespan by nearly one year. No significant effect was observed in women.
These findings remained consistent even after adjusting for related factors, including the role of phenylalanine. This suggests that tyrosine may independently influence aging. The researchers also observed that men tend to have higher tyrosine levels than women, which could partly explain the gender gap in lifespan.
“Phenylalanine showed no association with lifespan in either men or women after controlling for tyrosine.”
The exact mechanisms behind this effect are still under investigation. However, tyrosine’s involvement in insulin resistance and the production of stress-related neurotransmitters may be contributing factors. Insulin resistance is associated with many age-related diseases, and hormone-related pathways influenced by tyrosine may differ between men and women, potentially explaining the sex-specific outcomes.
Although tyrosine is commonly marketed as a supplement for enhancing focus and mental performance, the study raises concerns about its long-term impact on lifespan. While the researchers did not directly study tyrosine supplementation, their findings suggest that people with high tyrosine levels may benefit from dietary adjustments. Strategies such as protein restriction could help reduce tyrosine levels and support healthier aging.
Further studies are needed to confirm these findings and explore whether diet and lifestyle changes can safely lower tyrosine levels to promote longevity.
DOI - https://doi.org/10.18632/aging.206326
Corresponding author - Jie V. Zhao - janezhao@hku.hk
Abstract video - https://www.youtube.com/watch?v=rr0G44TD36M
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
To learn more about the journal, please visit https://www.Aging-US.com and connect with us on social media:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@Aging-US
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
Aging-US proudly sponsored the Future of Aging Research (FAR) Mixer 2025, hosted by the Aging Initiative on November 7 in Cambridge, MA, uniting students, researchers, and biotechnology leaders to advance aging research and shape a healthier, longer-lived future.
Highlights from the FAR Mixer 2025
The 2025 FAR Mixer featured keynote speaker Dr. Kristen Fortney, Co-Founder and CEO of BioAge Labs, who shared insights into how translational research and clinical pipelines have evolved over the past decade.
Dr. Fortney highlighted how obesity-targeting drugs are opening new avenues for metabolic and aging research. She explained that while obesity and osteoporosis are currently major therapeutic priorities, the next wave of reimbursable diseases will likely focus on muscle loss and chronic inflammation, reflecting their growing recognition as key factors in healthy aging.
She also emphasized the importance of human databases in target discovery, cross-sector partnerships between pharma and biotech, and the increasing focus on small-molecule interventions to address age-related diseases.
Focus talks showcased the diversity and depth of modern aging research.
Full recap - https://aging-us.org/2025/11/aging-us-supports-the-future-of-aging-research-mixer-2025/
To learn more about the journal, please visit www.Aging-US.com and connect with us on social media at:
Facebook - www.facebook.com/AgingUS/
X - twitter.com/AgingJrnl
Instagram - www.instagram.com/agingjrnl/
YouTube - www.youtube.com/@Aging-US
LinkedIn - www.linkedin.com/company/aging/
Bluesky - bsky.app/profile/aging-us.bsky.social
Pinterest - www.pinterest.com/AgingUS/
Spotify - open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
Synucleinopathies are a group of age-related neurological disorders, including Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Most individuals are not diagnosed until these diseases have significantly progressed, as early symptoms, such as a reduced sense of smell, subtle cognitive or motor changes are too vague to serve as reliable indicators.
To uncover specific biological signs that appear earlier and clearly point to the disease process, researchers from Saarland University developed a study titled “Brain region-specific and systemic transcriptomic alterations in a human alpha-synuclein overexpressing rat model,” featured as the cover Aging-US, Volume 17, Issue 10.
Full blog - https://aging-us.org/2025/11/alpha-synuclein-overexpression-in-rats-reveals-early-clues-to-synucleinopathies/
Paper DOI - https://doi.org/10.18632/aging.206331
Corresponding author - Thomas Hentrich - thomas.hentrich@uni-saarland.de
Abstract video - https://www.youtube.com/watch?v=Yl6AfVchkb0
Sign up for free Altmetric alerts about this article -
https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.206331
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
Keywords - aging, alpha-synuclein, transgenic rat model, different brain regions, transcriptome analysis
To learn more about the journal, please visit https://www.Aging-US.com and connect with us on social media at:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@Aging-US
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
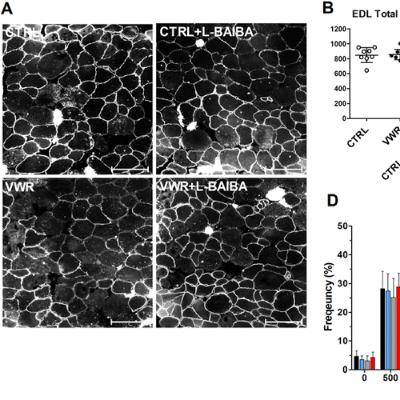
BUFFALO, NY — November 11, 2025 — A new #research paper was #published in Volume 17, Issue 10 of Aging-US on October 1, 2025, titled “L-β-aminoisobutyric acid (L-BAIBA) in combination with voluntary wheel running exercise enhances musculoskeletal properties in middle-age male mice.”
In this study led by first author Julian A. Vallejo and corresponding author Michael J. Wacker from the University of Missouri, Kansas City, researchers investigated how L-β-aminoisobutyric acid (L-BAIBA), a natural compound released during exercise, works together with regular physical activity to improve muscle and bone health in middle-aged male mice. The findings may support new strategies to maintain musculoskeletal health in aging populations, especially those at risk for mobility loss or osteoporosis.
Muscle and bone strength naturally decline with age, increasing the risk of falls, fractures, and reduced quality of life. While exercise remains the most effective way to counteract this deterioration, it is often difficult for older individuals to maintain sufficient activity levels to see results. L-BAIBA, a molecule naturally produced during physical activity, is known to promote energy metabolism and support muscle and bone cells. This study explored its potential to work in synergy with endurance exercise to maximize health benefits in aging bodies.
Researchers studied 12-month-old male mice that were split into different groups. Some remained sedentary, while others exercised freely on running wheels. Half of each group received daily L-BAIBA supplementation. After three months, the mice that received both the supplement and exercise showed greater improvements than those receiving either one alone. The soleus, a slow-twitch muscle essential for endurance and balance, grew larger and stronger only in the combined treatment group. These muscles also shifted to a more fatigue-resistant fiber type and had a larger number of oxidative fibers.
“To investigate this hypothesis, we subjected 12-month-old (as a model of middle-age) male C57BL6 mice to voluntary wheel running (VWR) with L-BAIBA (100mg/kg/day) (VWR+L-BAIBA), VWR alone, L-BAIBA alone, or none (CTRL) for three months.”
The study also showed significant improvements in bone health. Mice that received both exercise and L-BAIBA developed thicker and denser trabecular bone, along with reduced fat levels in the bone marrow, indicators of stronger, healthier bones. These changes were not observed in the groups that only exercised or only received L-BAIBA. Although the compound caused minor changes in heart electrical activity, it did not affect heart size or overall function, suggesting it is safe in this setting.
These findings suggest that L-BAIBA may enhance the benefits of physical activity by supporting muscle strength and bone structure, particularly in slow-twitch muscle fibers. This combination could serve as a therapeutic strategy to help older adults, including those unable to engage in regular exercise, maintain musculoskeletal health.
As the aging population grows, there is a growing need for solutions that support muscle and bone health without requiring strenuous activity. This research highlights the potential of natural, exercise-related molecules like L-BAIBA to help maintain mobility and strength throughout aging.
DOI - https://doi.org/10.18632/aging.206325
Corresponding author - Michael J. Wacker — wackerm@umkc.edu
Abstract video - https://www.youtube.com/watch?v=A-zfrLUikfQ
Visit https://www.Aging-US.com and connect with us on social media at:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@Aging-US
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
BUFFALO, NY — November 5, 2025 — A new #research paper was #published in Volume 17, Issue 10 of Aging-US on September 10, 2025, titled “Longitudinal associations of epigenetic aging with cognitive aging in Hispanic/Latino adults from the Hispanic Community Health Study/Study of Latinos.”
In this study led by Myriam Fornage, from The University of Texas Health Science Center at Houston, researchers found that faster biological aging, measured by DNA-based epigenetic clocks, is associated with greater cognitive decline and higher risk of mild cognitive impairment (MCI) in Hispanic/Latino adults. The results highlight the potential of epigenetic clocks to track changes in brain health over time, helping improve early detection and monitoring of age-related cognitive problems.
Cognitive decline and dementia are major public health concerns, especially among aging populations. In this study, researchers followed 2671 Hispanic/Latino adults (average age 57; 66% women) over a seven-year period. They measured each participant’s biological age using epigenetic clocks and assessed their cognitive performance at two time points.
“We evaluated the associations of 5 epigenetic clocks and their between-visit change with multiple measures of cognitive aging that included a global and domain-specific cognitive function score at each visit, between-visit change in global and domain-specific cognitive function score, and MCI diagnosis at visit 2 (V2).”
Epigenetic clocks estimate biological age based on DNA chemical modifications, called methylation, that accumulate with age. The study evaluated five different clocks, including newer models like GrimAge and DunedinPACE, which are designed to more accurately reflect health-related aging.
The researchers found that individuals with faster biological aging showed lower cognitive function and higher probability of developing MCI over time. Among the five clocks studied, newer models such as GrimAge and DunedinPACE showed the strongest associations with memory, processing speed, and overall brain health. These findings suggest that tracking changes in biological age over time may be more effective than relying on a single measurement to identify those at risk for cognitive impairment.
Importantly, the associations between biological aging and cognitive decline remained significant even after accounting for other known risk factors such as education, language preference, and cardiovascular health. This supports the idea that epigenetic clocks capture unique biological processes that influence brain aging. The study also found that the impact of changes in biological age over time was comparable to that of APOE4, a well-established genetic risk factor for Alzheimer’s disease.
Overall, this is the first large-scale study to examine these associations in a Hispanic/Latino population, a group that is underrepresented in aging research. By identifying early biological signs of brain aging, this work highlights the potential of epigenetic clocks as tools for routine health assessments. Monitoring changes in these biological markers could help detect individuals at risk for cognitive decline and guide timely interventions to preserve brain health.
DOI - https://doi.org/10.18632/aging.206317
Corresponding author - Myriam Fornage - Myriam.Fornage@uth.tmc.edu
Abstract video - https://www.youtube.com/watch?v=kG0Y-F_sods
To learn more about the journal, please visit https://www.Aging-US.com and connect with us on social media:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@Aging-US
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
BUFFALO, NY — November 3, 2025 — A new #research paper featured on the #cover of Volume 17, Issue 10 of Aging-US was #published on October 20, 2025, titled “Brain region-specific and systemic transcriptomic alterations in a human alpha-synuclein overexpressing rat model.”
In this study, led by first author Vivien Hoof and corresponding author Thomas Hentrich from Saarland University, Germany, researchers investigated how excess alpha-synuclein—a protein linked to Parkinson’s disease—affects gene activity in different brain regions and the gut. They found that early, region-specific gene disruptions may contribute to the appearance of disease, with some effects also detected in the gut. These early molecular changes could serve as biomarkers for Parkinson’s and point to new directions for treatment.
Alpha-synuclein accumulates in the brains of individuals with Parkinson’s disease and other age-related neurological conditions known as synucleinopathies. To better understand this process, the research team used a genetically modified rat model that overexpresses human alpha-synuclein. They studied gene expression in the striatum, cortex, and cerebellum—three key brain regions involved in movement and cognition—and analyzed how these changes evolved with age.
“Transcriptomic analyses were performed on gene and transcript level of striatal, frontocortical, and cerebellar tissue in 5- and 12-month-old transgenic (BAC SNCA) and wild type rats […]”
The results showed that gene alterations appeared earlier and were more pronounced in young rats, particularly in the striatum and cortex, before any visible signs of disease manifested. This early disruption challenges the common belief that gene alterations gradually increase with age and suggests that early-life molecular changes may be critical in disease development.
The researchers also found that many gene expression changes were unique to individual brain regions. However, they identified a set of genes that were consistently affected across all brain regions and the gut. This suggests that the disease may begin to affect the entire body—not just the brain—long before symptoms become noticeable.
Several of the shared genes are involved in synaptic signaling and inflammation—processes known to be altered in Parkinson’s. Others are linked to dopamine production and neuronal plasticity, indicating potential early efforts by the brain to compensate for the harmful effects of the alpha-synuclein buildup.
Overall, this study provides a detailed view of how alpha-synuclein affects gene networks early in the disease process. Understanding these changes may help identify biomarkers and develop targeted therapies before irreversible brain damage occurs.
DOI - https://doi.org/10.18632/aging.206331
Corresponding author - Thomas Hentrich - thomas.hentrich@uni-saarland.de
Abstract video - https://www.youtube.com/watch?v=Yl6AfVchkb0
Sign up for free Altmetric alerts about this article -
https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.206331
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
Keywords - aging, alpha-synuclein, transgenic rat model, different brain regions, transcriptome analysis
To learn more about the journal, please visit https://www.Aging-US.com and connect with us on social media at:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@Aging-US
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
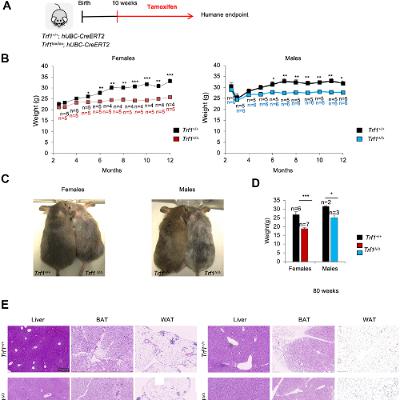
BUFFALO, NY — October 27, 2025 — A new #research paper was #published in Volume 17, Issue 9 of Aging-US on September 17, 2025, titled “Depletion of the TRF1 telomere-binding protein leads to leaner mice with altered metabolic profiles.”
In this study led by first author Jessica Louzame Ruano and corresponding author Maria A. Blasco from the Spanish National Cancer Centre (CNIO), researchers investigated the role of TRF1, a protein known for protecting telomeres, in regulating whole-body metabolism. The results suggest that TRF1 influences metabolic health through mechanisms unrelated to its known function in telomere maintenance.
Obesity and metabolic disorders are major health concerns, especially as people age. To explore TRF1’s role beyond telomere protection, the research team studied both normal mice and genetically modified mice that lacked TRF1. Mice without TRF1 remained leaner over time, resisted fat accumulation, and showed healthier blood sugar and insulin levels compared to normal mice. Importantly, these benefits occurred without any detectable shortening of telomeres.
The leaner body composition in TRF1-deficient mice was not due to reduced food intake or increased physical activity. Instead, the fat loss appeared to result from biological changes in how energy was processed and stored. Male mice without TRF1 gained less weight and had lower LDL cholesterol levels, even on a high-fat diet. Female mice showed milder effects, reflecting known sex-based differences in susceptibility to diet-induced obesity. This highlights the importance of including both sexes in metabolic research.
“Major metabolic pathways related with energy production and regulation of metabolism homeostasis were also found downregulated in Trf1-deficient mice.”
Gene expression analysis in the liver revealed shifts in several key pathways. Genes related to fat production, energy generation, and muscle growth were downregulated, while genes linked to inflammation and cholesterol synthesis were upregulated. The mice also showed signs of higher energy expenditure and a shift from using fat to protein as an energy source, possibly due to their reduced fat reserves. However, some older mice developed mild liver stress, including fibrosis and DNA damage, suggesting a possible long-term trade-off.
Overall, this study expands the understanding of how telomere-related proteins influence more than just cellular aging. By identifying a connection between TRF1 and metabolism, the research opens new possibilities for targeting TRF1 or its pathways to address obesity and related conditions. Still, further studies are needed to clarify how TRF1 affects fat development and whether similar effects occur in humans.
DOI - https://doi.org/10.18632/aging.206320
Corresponding author - Maria A. Blasco — mblasco@cnio.es
Abstract video - https://www.youtube.com/watch?v=7AG3TBgDZIw
Sign up for free Altmetric alerts about this article -
https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.206320
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
Keywords - aging, Trf1, metabolism, leaner, fat, telomeres
To learn more about the journal, visit https://www.Aging-US.com and connect with us on social media at:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@AgingJournal
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
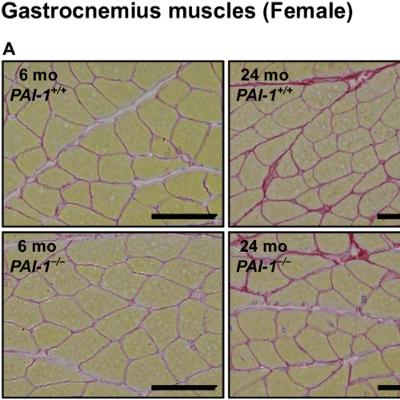
BUFFALO, NY — October 23, 2025 — A new #research paper was #published in Volume 17, Issue 9 of Aging-US on September 11, 2025, titled “Roles of plasminogen activator inhibitor-1 in aging-related muscle and bone loss in mice.”
In this study led by first author Takashi Ohira and corresponding author Hiroshi Kaji from Kindai University Faculty of Medicine, researchers found that female mice lacking the gene for plasminogen activator inhibitor-1 (PAI-1) were protected from age-related muscle weakness and bone thinning. This suggests that PAI-1 could be a potential target for future treatments to reduce frailty in aging populations.
As the global population continues to age, more people are affected by conditions such as sarcopenia and osteoporosis. These disorders involve the progressive loss of skeletal muscle mass and bone density, leading to reduced mobility, a greater risk of falls, and a lower quality of life.
To investigate the role of PAI-1 in aging, researchers compared young (6-month-old) and aged (24-month-old) male and female mice, with and without the PAI-1 gene. They found that PAI-1 levels increased with age in both sexes. However, only female mice lacking the PAI-1 gene experienced a significant reduction in age-related muscle and bone loss.
Female mice without PAI-1 maintained stronger grip strength and greater muscle mass in their lower limbs. They also showed less cortical bone loss in their femurs and tibias. In contrast, male mice did not experience the same benefits, despite also showing increased levels of PAI-1 with age. These results suggest that PAI-1 contributes to aging-related decline in a sex-specific manner.
“The present study found that lower limb muscle mass, gastrocnemius and soleus muscle tissue weights, and grip strength were significantly lower in 24-month-old male and female wild-type mice than in their 6-month-old counterparts.”
PAI-1 plays key roles in blood clotting, inflammation, and cellular senescence—a process in which aging cells release harmful molecules that affect nearby tissues. One of these molecules, interleukin-6 (IL-6), is a major driver of inflammation. The researchers found that aged female mice lacking PAI-1 had lower IL-6 levels in both muscle and blood, suggesting that PAI-1 may contribute to muscle and bone loss by promoting inflammation. These protective effects were also not associated with changes in muscle protein turnover or reductions in fibrous tissue, reinforcing the idea that PAI-1’s impact is likely driven by inflammatory signaling.
This study highlights PAI-1 as a promising therapeutic target for slowing or preventing age-related declines in muscle and bone health, particularly in women. Since postmenopausal women are especially vulnerable to osteoporosis and frailty, a better understanding of how PAI-1 contributes to aging could lead to new strategies for maintaining strength and mobility in later life. Further research is needed to explore how PAI-1 interacts with other age-related biological changes and why its effects differ between sexes.
DOI - https://doi.org/10.18632/aging.206318
Corresponding author - Hiroshi Kaji - hkaji@med.kindai.ac.jp
Abstract video - https://www.youtube.com/watch?v=hg4qKf-oO2I
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
To learn more about the journal, please visit https://www.Aging-US.com and connect with us on social media at:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@Aging-US
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
BUFFALO, NY — October 21, 2025 — A new #editorial was #published in Aging-US on October 13, 2025, titled “Longevity clinics: between promise and peril.”
In this editorial, Marco Demaria, Editor-in-Chief of Aging-US, from the European Research Institute for the Biology of Ageing (ERIBA), University Medical Center Groningen (UMCG), University of Groningen (RUG), reviews the rapid rise of longevity clinics worldwide.
Longevity clinics have emerged globally in response to increasing demand for personalized, preventive healthcare. Located in countries such as the United States, Switzerland, and the United Arab Emirates, these centers offer advanced diagnostic services, including genomic testing, advanced imaging, and multi-omics profiling. Their goal is to extend healthspan—the number of years a person lives in good health—through customized lifestyle interventions, nutritional guidance, and, in some cases, experimental therapies.
“Longevity clinics embody an important vision: healthcare is personalized, preventive, and engaged.”
Although the concept of proactive aging care is attractive, the editorial raises serious concerns about the scientific and ethical foundations of these clinics. Many operate outside conventional medical systems and lack connections to academic geroscience. This disconnection allows them to market expensive interventions without sufficient clinical validation. Program costs can range from €10,000 to over €100,000 per year, limiting access to wealthy individuals while leaving out populations most at risk for premature aging.
Despite these challenges, Dr. Demaria notes that longevity clinics may contribute meaningfully to innovation. By collecting extensive, long-term health data from clients, these clinics have the potential to identify early biomarkers of aging and detect signs of age-related diseases. Unlike traditional clinical trials, which are limited in scope and duration, longevity clinics track a wide range of health data over time. When paired with artificial intelligence tools, this information could help advance the science of healthy aging.
However, several risks remain. Many clinics lack standardized protocols, and the tools they use, such as biological age calculators or hormone therapies, often lack accuracy or clear clinical value. Without proper guidelines, clients may receive advice that is confusing or not scientifically supported. This can reduce public trust in the broader field of longevity research.
To ensure these clinics contribute positively to health innovation, the editorial outlines different key steps: greater collaboration with academic researchers, the adoption of standardized protocols, increased transparency, and work toward regulatory clarity. Broader access must also be considered by developing scalable and more affordable models, possibly through partnerships with public health systems.
Ultimately, longevity clinics represent both a major opportunity and a serious concern. If integrated responsibly with science, policy, and public health, they could support a shift toward personalized, preventive healthcare. Without this alignment, however, they risk reinforcing inequality and weakening the credibility of the science behind aging.
DOI - https://doi.org/10.18632/aging.206330
Corresponding author - Marco Demaria — m.demaria@umcg.nl
Abstract video - https://www.youtube.com/watch?v=Bt84xBdii0s
To learn more about the journal, visit https://www.Aging-US.com and connect with us on social media at:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@AgingJournal
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
BUFFALO, NY — October 17, 2025 — A new #research paper was #published in Volume 17, Issue 9 of Aging-US on September 8, 2025, titled, “Runx1 overexpression induces early onset of intervertebral disc degeneration.”
In this study, led by first author Takanori Fukunaga from Emory University School of Medicine and corresponding author Hicham Drissi from Emory and the Atlanta VA Medical Center, researchers found that the Runx1 gene, when overactive in spinal disc cells, can accelerate age-related degeneration of the intervertebral discs. The findings offer new insight into the genetic factors that drive disc aging and suggest possible directions for treating chronic back pain.
Intervertebral discs cushion the spine and support movement. Their deterioration is a major cause of lower back pain, especially with aging. At the center of each disc is the nucleus pulposus (NP), a gel-like core that contains proteins such as collagen and aggrecan, which help retain water and maintain structure. As people age, NP cells often lose their function, contributing to disc breakdown.
Using a genetically modified mouse model, the researchers activated Runx1 specifically in NP cells. These mice developed signs of disc degeneration by five months of age, which is much earlier than normal. The overexpression of Runx1 led to the loss of healthy NP cells, an increase in abnormal cell types, and damage to disc structure. Levels of essential proteins like aggrecan and type II collagen decreased, while type X collagen increased, signaling unhealthy tissue changes.
“To achieve NP-specific postnatal overexpression of Runx1, we crossed Krt19CreERT mice with Rosa26-Runx1 transgenic mice previously generated in our laboratory.”
A key finding was that Runx1 overactivity did not kill cells directly. Instead, it caused premature cellular aging, known as senescence. Senescent cells lose the ability to repair tissue, creating an environment that accelerates degeneration. Markers of senescence were significantly elevated in the affected discs.
The researchers also observed a dose-dependent response. The more Runx1 was activated, the more severe the degeneration was. This suggests that targeting Runx1 may be a promising strategy to prevent or slow disc aging.
Overall, this study highlights the genetic and cellular processes that contribute to intervertebral disc degeneration, a leading cause of disability. By identifying Runx1 as a potential driver of early disc aging, the research opens new opportunities for intervention and treatment of degenerative spine conditions.
DOI - https://doi.org/10.18632/aging.206316
Corresponding author - Hicham Drissi - hicham.drissi@emory.edu
Abstract video - https://www.youtube.com/watch?v=BPwWbVBPIUM
Sign up for free Altmetric alerts about this article -
https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.206316
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
Keywords - cell senescence, aging, Runx1, nucleus pulposus, intervertebral disc degeneration
To learn more about the journal, please visit our website at https://www.Aging-US.com and connect with us on social media at:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@Aging-US
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
As people age, it is common to experience some memory lapses or slower thinking. Although this is often a normal part of aging, it can still affect a person’s quality of life. Scientists have been investigating ways to slow or prevent cognitive decline, and growing evidence points to the potential role of social interaction.
Recently, a study using rats found that long-term social connection may help protect the brain from age-related memory decline. This work, titled “The impact of long-term social housing on biconditional association task performance and neuron ensembles in the anterior cingulate cortex and the hippocampal CA3 region of aged rats,” was recently published in Aging-US (Volume 17, Issue 9).
Full blog - https://aging-us.org/2025/10/how-long-term-social-connection-supports-brain-health-and-memory-in-aging/
Paper DOI - https://doi.org/10.18632/aging.206310
Corresponding author - Anne M. Dankert - adankert@unc.edu
Abstract video - https://www.youtube.com/watch?v=poNnPz1ti6Q
Sign up for free Altmetric alerts about this article -
https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.206310
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
Keywords - aging, aging, environmental enrichment, working memory, complex cognition, immediate early genes
To learn more about the journal, please visit our website at https://www.Aging-US.com and connect with us on social media at:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@AgingJournal
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
BUFFALO, NY — October 14, 2025 — A new #research paper was #published in Volume 17, Issue 9 of Aging-US on August 30, 2025, titled, “Glycocalyx-targeted therapy prevents age-related muscle loss and declines in maximal exercise capacity.”
In this study, led by Daniel R. Machin from the University of New Mexico School of Medicine and the University of Utah, researchers found that protecting a fragile layer lining blood vessels, known as the glycocalyx, can prevent muscle deterioration and help maintain physical performance during aging. They also discovered that a supplement containing high-molecular-weight hyaluronan (HMW-HA), a key component of the glycocalyx, enabled older mice to preserve muscle mass and exercise capacity. These findings suggest that targeting the glycocalyx may offer a new approach to reduce frailty and support mobility in older adults.
As this layer degrades with age, it contributes to cardiovascular and muscular decline by impairing blood flow and vascular health. The study examined how preserving the glycocalyx using a therapy called Endocalyx™ affects physical function in aging mice.
Researchers first studied genetically modified mice lacking Has2, the enzyme responsible for producing HMW-HA. These mice had a thinner glycocalyx, reduced exercise performance, and lower mitochondrial function in their muscles, even though muscle size remained normal. This indicated that glycocalyx damage alone can directly impair physical performance.
The team then gave older mice a diet containing Endocalyx™ for 10 weeks. Compared to untreated controls, these mice maintained muscle mass and performed better on treadmill tests. Notably, the treated mice did not show the typical age-related decline in muscle strength and endurance. While the supplement did not fully restore youthful performance, it significantly slowed physical deterioration, suggesting a protective benefit. In contrast, untreated older mice lost both body mass and muscle volume during the same period.
“Taken together, these findings provide direct evidence of a role for HMW-HA in the modulation of exercise capacity.”
This research builds on prior evidence that the glycocalyx is essential for healthy blood vessel function. Since muscle health depends on proper blood flow and oxygen delivery, restoring the glycocalyx may help maintain strength and mobility with age. While more research is needed to confirm these results in humans, the findings point to a potential therapeutic approach to promote healthier aging.
DOI - https://doi.org/10.18632/aging.206313
Corresponding author - Daniel R. Machin — dmachin@salud.unm.edu
Abstract video - https://www.youtube.com/watch?v=S7HjCeXT8fU
Sign up for free Altmetric alerts about this article -
https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.206313
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
Keywords - aging, glycocalyx, hyaluronan
To learn more about the journal, please visit our website at https://www.Aging-US.com and connect with us on social media at:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@AgingJournal
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
Federica Grosso from the Institute for Genetic and Biomedical Research (IRGB) of the National Research Council (CNR) in Monserrato, Italy, describes a #research paper she co-authored that was #published in Volume 17, Issue 8 of Aging-US, entitled “Causal relationships between gut microbiome and hundreds of age-related traits: evidence of a replicable effect on ApoM protein levels.”
DOI - https://doi.org/10.18632/aging.206293
Corresponding author - Serena Sanna - serena.sanna@cnr.it
Video interview - https://www.youtube.com/watch?v=qYg42_gn_pw
Abstract
In the past 20 years, the involvement of gut microbiome in human health has received particular attention, but its contribution to age-related diseases remains unclear. To address this, we performed a comprehensive two-sample Mendelian Randomization investigation, testing 55130 potential causal relationships between 37 traits representing gut microbiome composition and function and age-related phenotypes, including 1472 inflammatory and cardiometabolic circulating plasma proteins from UK Biobank Pharma Proteomic Project and 18 complex traits. A total of 91 causal relationships remained significant after multiple testing correction (false discovery rate p-value <0.05) and sensitivity analyses, notably two with the risk of developing age-related macular degeneration and 89 with plasma proteins. The link between purine nucleotides degradation II aerobic pathway and apolipoprotein M was further replicated using independent genome-wide association study data. Finally, by taking advantage of previously reported biological function of Faecalibacterium prausnitzii we found evidence of regulation of six proteins by its function as mucosal-A antigen utilization. These results support the role of gut microbiome as modulator of the inflammatory and cardiometabolic circuits, that may contribute to the onset of age-related diseases, albeit future studies are needed to investigate the underlying biological mechanisms.
Sign up for free Altmetric alerts about this article -
https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.206293
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
Keywords - aging, causal inference, aging, gut microbiome, inflammatory proteins, age-related macular degeneration
To learn more about the journal, please visit our website at https://www.Aging-US.com and connect with us on social media at:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@AgingJournal
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
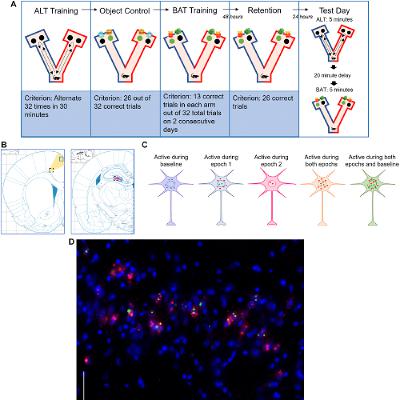
BUFFALO, NY — October 9, 2025 — A new #research paper was #published in Volume 17, Issue 9 of Aging-US on August 22, 2025, titled, “The impact of long-term social housing on biconditional association task performance and neuron ensembles in the anterior cingulate cortex and the hippocampal CA3 region of aged rats.”
The research team led by Anne M. Dankert from Providence College and University of North Carolina, Chapel Hill, showed that aged rats who lived in socially enriched environments throughout life retained better memory and cognitive flexibility than those housed alone. This study highlights the importance of social interaction in protecting the aging brain.
Cognitive decline, such as memory loss and reduced problem-solving ability, affects many people over the age of 65. While many factors contribute to age-related cognitive decline, this study suggests that one key factor may be surprisingly simple: long-term social connection. To explore how social interaction might influence memory performance and brain activity, the researchers designed a study using rats as a model for aging in humans.
“Cognitive decline and changes in neuronal activity are hallmarks of aging.”
They compared three groups of rats: young adults, aged rats housed alone, and aged rats housed socially in groups. All groups had access to the same physical enrichment, such as exercise and stimulating objects, but only some experienced lifelong social companionship. The team tested these animals on a complex memory challenge known as the biconditional association task, which requires animals to make context-based decisions—an ability that typically declines with age.
The results showed that aged rats living in social groups performed just as well as young adults on the memory task, while those housed alone showed significant impairments. Socially housed rats also made fewer working memory errors and required less effort to complete cognitive tasks, suggesting not only better performance but more efficient brain function. These benefits were not observed in aged rats who received only environmental enrichment without social interaction.
Brain imaging revealed additional differences between the groups. Socially housed aged rats showed increased activity in the hippocampus, particularly in the CA3 region, which plays a key role in forming and separating memories. In contrast, aged rats that lived alone had lower activity in this region, which may explain their poorer performance. Interestingly, socially housed rats also showed reduced overactivity in the anterior cingulate cortex—a brain area involved in attention and decision-making—suggesting a more balanced and efficient neural response.
This research provides new insight into how lifelong social experiences shape brain health during aging. While earlier studies have shown that physical activity and cognitive stimulation help preserve cognitive function, this study identifies social interaction as an independent and powerful protective factor. The findings are consistent with human studies showing that older adults who remain socially active tend to experience slower cognitive decline and stronger brain function.
Overall, these results emphasize that brain aging is not inevitable but may be influenced by our social environments. This research suggests that fostering lifelong social connections could be a critical, low-cost strategy to protect memory and mental flexibility in older adults.
DOI - https://doi.org/10.18632/aging.206310
Corresponding author - Anne M. Dankert - adankert@unc.edu
Abstract video - https://www.youtube.com/watch?v=poNnPz1ti6Q
https://www.aging-us.com/
MEDIA@IMPACTJOURNALS.COM
Dr. Leonard Egede, Dr. Rebekah Walker, and Dr. Obinna Ekwunife from the Department of Medicine at the University of Buffalo, NY, describe their #research paper #published in Volume 17, Issue 8 of Aging-US, entitled “Longitudinal relationship between social and CVD risk factors in older adults with prediabetes: the HRS 2006-2016.”
#interview #authorinterview #aging #prediabetes #cardiovascular #health #openaccess #openscience #peerreviewed #journal #publication #publishing #meded
DOI - https://doi.org/10.18632/aging.206308
Corresponding author - Leonard E. Egede - legede@buffalo.edu
Video interview - https://www.youtube.com/watch?v=1MSTk3GQAGA
Video transcript - https://aging-us.net/2025/10/08/behind-the-study-social-and-cardiovascular-risk-factors-in-older-adults-with-prediabetes/
Abstract
Background: This study examines how multiple social risk factors influence cardiovascular disease (CVD) risk control over time in older adults with prediabetes using a nationally representative cohort.
Methods: Data from the Health and Retirement Study (HRS) included 5,086 U.S. adults aged 50+ with prediabetes. Five social risk domains (economic stability, environment, education, healthcare, and social context) were examined as independent variables, while CVD risk factors included glycemic control (HbA1c), systolic blood pressure (SBP), and cholesterol ratio (total cholesterol/high-density lipoprotein). Mixed-effects models assessed relationships between social risk factors and CVD outcomes, adjusting for age, gender, race, and marital status.
Results: The sample had an average age of 68.6 years, with 60.2% female, and 70.97% identifying as non-Hispanic Black. Average HbA1c was 5.7, SBP 129.4, and cholesterol ratio 3.85. Limited education was consistently associated with increased CVD risk—HbA1c (β = 0.03, 95% CI: 0.01–0.06, p < 0.001), SBP (β = 4.34, 95% CI: 2.96–5.71, p < 0.001), and cholesterol ratio (β = 0.08, 95% CI: 0.01–0.16, p < 0.05) —in the fully adjusted model. Medication cost-related non-adherence was significantly associated with higher HbA1c levels (β = 0.03, 95% CI: 0.002–0.06, p < 0.05). Difficulty paying bills and lack of health insurance were both significantly associated with higher cholesterol levels (β = 0.03, 95% CI: 0.002–0.06, p < 0.05) and (β = 0.22, 95% CI: 0.15–0.30, p < 0.001), respectively.
Conclusions: Social risk factors, particularly limited education, significantly impact CVD risk in older adults with prediabetes.
Sign up for free Altmetric alerts about this article -
https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.206308
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
Keywords - aging, prediabetes, social determinants of health, health equity, cardiovascular health, population health
To learn more about the journal, visit https://www.Aging-US.com and connect with us on social media at:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@AgingJournal
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
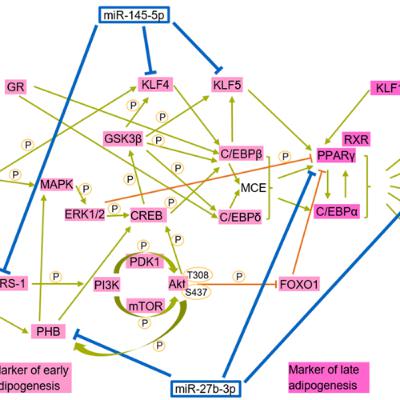
BUFFALO, NY — October 7, 2025 — A new #research paper was #published in Volume 17, Issue 9 of Aging-US on August 27, 2025, titled, “Deregulated miR-145 and miR-27b in Hutchinson-Gilford progeria syndrome: implications for adipogenesis.”
In this study, led by first author Felix Quirin Fenzl and corresponding author Karima Djabali from the Technical University of Munich (TUM), researchers identified that miR-145-5p and miR-27b-3p interfere with the formation of fat cells in children with Hutchinson-Gilford progeria syndrome (HGPS), a rare and fatal premature aging disorder. Their findings help explain why patients often experience fat loss and related metabolic complications and suggest new potential therapeutic strategies.
Hutchinson-Gilford progeria syndrome is a genetic condition that causes rapid aging in children, often leading to early death due to heart disease. Although affected children appear healthy at birth, they soon develop signs of accelerated aging, including hair loss, stiff joints, and a significant reduction in fat tissue. While certain treatments can slow disease progression, many aspects, such as the loss of fat tissue, remain poorly understood.
“Overall, this study provides the first comprehensive miRNA profiling of HGPS and control fibroblasts across different stages of cellular senescence.”
This study focused on how microRNAs—tiny molecules that help regulate gene expression—contribute to the disease. To explore this, the researchers used skin-derived stem cells from both healthy individuals and HGPS patients. When they transformed these cells into fat cells, the HGPS-derived stem cells formed significantly fewer fat cells. This difference was linked to unusually high levels of miR-145-5p and miR-27b-3p. These molecules were found to silence important genes required for fat cell growth and function. When the researchers blocked these microRNAs, fat cell formation improved.
The team also examined fat tissue from a mouse model of HGPS. Similar to the human cells, these mice showed increased levels of miR-145-5p and miR-27b-3p and impaired fat development. These results confirm that these two microRNAs play a central role in the loss of fat tissue seen in the disease. Importantly, reducing their activity could become a promising therapeutic strategy for restoring fat tissue in affected individuals.
Although further research is needed before developing treatments, this study represents a step forward in understanding the molecular causes of lipodystrophy, a condition in which the body cannot form healthy fat tissue, in HGPS. It also opens the door for future therapies that could improve quality of life and health outcomes for patients. In the long term, similar approaches might benefit people with other metabolic diseases, such as obesity or diabetes, where fat cell function is also disrupted.
DOI - https://doi.org/10.18632/aging.206309
Corresponding authors - Karima Djabali — djabali@tum.de
Abstract video - https://www.youtube.com/watch?v=b0ksC3cvdZ0
Sign up for free Altmetric alerts about this article -
https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.206309
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
Keywords - aging, Hutchinson-Gilford progeria syndrome (HGPS), progerin, microRNAs, adipogenesis
To learn more about the journal, please visit our website at https://www.Aging-US.com and connect with us on social media at:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@AgingJournal
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
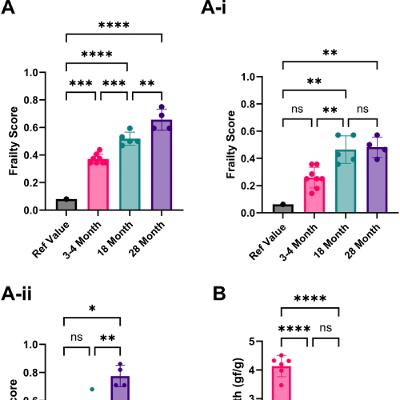
BUFFALO, NY — October 3, 2025 — A new #research perspective was #published in Volume 17, Issue 9 of Aging-US on August 26, 2025, titled “Analysis of the current state of frailty indexes and their implementation for aging intervention studies.”
In this work, led by first author Oliver G. Frost from Loughborough University alongside corresponding authors Abdelhadi Rebbaa and Amit Sharma, from the Lifespan Research Institute, the authors explore growing concerns about the lack of standardization in how frailty is measured in rodent aging studies, which may limit the development of effective interventions targeting age-related decline.
Frailty, a key indicator of deteriorating health in older adults, is increasingly assessed in preclinical models using frailty indexes (FIs). These indexes quantify health deficits, such as reduced mobility, cognitive decline, or physical weakness. However, this perspective highlights that FI methodologies vary significantly across studies, from the selection of parameters to the cut-off thresholds used, resulting in inconsistent outcomes that affects reproducibility and translational value.
The authors reviewed 18 rodent studies and found substantial variation in how frailty is defined and measured. Some FIs rely on clinical observations, such as appearance or beahaviour, while others focus on physical performance metrics like grip strength or locomotion. In several cases, applying different FIs to the same group of animals produced contradictory results, underscoring the importance of harmonized protocols.
To illustrate these issues, the researchers applied an 8-item FI to mice of different ages and found that even young mice were sometimes scored as frail, depending on the scoring method and reference values. This finding emphasizes the need for consistent baselines and controlled environments, especially when comparing across studies. The authors recommend using each animal as its own baseline in longitudinal studies, a strategy that enhances reliability without adding significant cost.
“Sex as a biological variable in FIs is an important consideration, as there is a known difference between male and female frailty onset and progression.”
The authors also discuss emerging automated tools, such as video-based open-field testing, which can reduce observer bias and improve reproducibility. In the future, broader health indicators, such as cognition, circadian rhythms, social behavior, and body composition, may further enhance frailty assessments.
Overall, this work underscores the urgent need for standardized, transparent, and reproducible methods for evaluating frailty in preclinical aging studies. Improved consistency in frailty scoring will better inform the development of healthspan-extending therapies and enhance the translational relevance of animal models.
DOI - https://doi.org/10.18632/aging.206307
Corresponding authors - Abdelhadi Rebbaa - rebbaa@gmail.com, and Amit Sharma - amit.sharma@sens.org
Abstract video - https://www.youtube.com/watch?v=eha3XA9LyWA
Sign up for free Altmetric alerts about this article -
https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.206307
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
Keywords - aging, frailty, rodents, frailty index, phenotype
To learn more about the journal, please visit our website at https://www.Aging-US.com and connect with us on social media at:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@AgingJournal
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
As life expectancy increases, there is growing interest not only in extending lifespan but also in improving the quality of those additional years. To address the physical and cognitive decline that often accompanies aging, researchers have explored a variety of strategies. Many of these focus on a single biological factor, such as reducing inflammation or stimulating stem cell activity. However, aging is a complex process involving multiple, interconnected changes in the body.
Recognizing this, researchers at the University of California, Berkeley proposed a more comprehensive approach: targeting multiple aging-related pathways simultaneously. Their study, titled “Sex-specific longitudinal reversal of aging in old frail mice,” was recently featured on the cover of Aging-US (Volume 17, Issue 9).
Full blog - https://aging-us.org/2025/10/new-anti-aging-combo-boosts-lifespan-in-old-male-mice/
Paper DOI - https://doi.org/10.18632/aging.206304
Corresponding author - Irina M. Conboy - irina@generationlab.co
Abstract video - https://www.youtube.com/watch?v=bpWxDd7hHhM
Sign up for free Altmetric alerts about this article -
https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.206304
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
Keywords - aging, lifespan, healthspan, Alk5 inhibitor, oxytocin, sex-specific differences
To learn more about the journal, please visit our website at https://www.Aging-US.com and connect with us:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@AgingJournal
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
BUFFALO, NY — October 1, 2025 — A new #research paper #featured as the #cover of Volume 17, Issue 9 of Aging-US was published on August 21, 2025, titled “Sex-specific longitudinal reversal of aging in old frail mice.”
The study, led by first author Cameron Kato and corresponding author and Aging-US Editorial Board Member Irina M. Conboy from the University of California, Berkeley, reports that a combination of oxytocin and an Alk5 inhibitor (OT+A5i) significantly extended both lifespan and healthspan in frail, elderly, male mice. These rejuvenating effects were not seen in female mice, highlighting key biological differences between the sexes in their response to aging therapies.
The researchers tested a dual-drug approach targeting two biological pathways that change with age. Oxytocin, a hormone that declines with aging and supports tissue repair, was combined with an Alk5 inhibitor that blocks the TGF-beta pathway. TGF-beta becomes overactive with age and contributes to chronic inflammation and tissue damage. In this study, frail mice at 25 months of age—roughly equivalent to 75 human years—were treated regularly with the OT+A5i combination.
Male mice receiving the therapy lived over 70% longer than untreated controls and showed significant improvements in physical endurance, agility, and memory. According to hazard ratio analysis, the treated males were nearly three times less likely to die at any given time than untreated males.
“Treatment of old frail male mice with OT+A5i resulted in a remarkable 73% life extension from that time, and a 14% increase in the overall median lifespan.”
The therapy also reduced “biological noise” in circulating blood proteins—an established marker of aging—bringing those levels back to a more youthful state. Short-term benefits, were seen in both sexes, however, after four months of continuous treatment, only the male mice showed sustained improvement in systemic protein balance. Female mice did not experience significant gains in lifespan or healthspan, though middle-aged females did show improved fertility after treatment.
These results underscore the importance of understanding sex-specific biology when developing treatments for aging. While the reasons for these differences remain unclear, the findings provide a new model for studying and designing longevity therapies.
Oxytocin is already FDA-approved, and Alk5 inhibitors are currently in clinical trials, suggesting that this approach could be translated to humans. With strong results in aged and frail male animals, OT+A5i appears to be a promising candidate for improving late-life health and survival.
DOI - https://doi.org/10.18632/aging.206304
Corresponding author - Irina M. Conboy - irina@generationlab.co
Abstract video - https://www.youtube.com/watch?v=bpWxDd7hHhM
Sign up for free Altmetric alerts about this article -
https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.206304
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
Keywords - aging, lifespan, healthspan, Alk5 inhibitor, oxytocin, sex-specific differences
To learn more about the journal, please visit our website at https://www.Aging-US.com and connect with us:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@AgingJournal
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM
In this episode of the Longevity & Aging Series, Girish Harinath from AgelessRx joins host Dr. Evgeniy Galimov to discuss a research paper he co-authored in Volume 17, Issue 4 of Aging-US, titled “Influence of rapamycin on safety and healthspan metrics after one year: PEARL trial results.”
DOI - https://doi.org/10.18632/aging.206235
Corresponding author - Stefanie L. Morgan - stefanie@agelessrx.com
Video interview - https://www.youtube.com/watch?v=7-NvskI8Ve0
Longevity & Aging Series - https://www.aging-us.com/longevity
Abstract
Design: This 48-week decentralized, double-blinded, randomized, placebo-controlled trial (NCT04488601) evaluated the long-term safety of intermittent low-dose rapamycin in a healthy, normative-aging human cohort. Participants received placebo, 5 mg or 10 mg compounded rapamycin weekly. The primary outcome measure was visceral adiposity (by DXA scan), secondary outcomes were blood biomarkers, and lean tissue and bone mineral content (by DXA scan). Established surveys were utilized to evaluate health and well-being. Safety was assessed through adverse events and blood biomarker monitoring.
Results: Adverse and serious adverse events were similar across all groups. Visceral adiposity did not change significantly (ηp2 = 0.001, p = 0.942), and changes in blood biomarkers remained within normal ranges. Lean tissue mass (ηp2 = 0.202, p = 0.013) and self-reported pain (ηp2 = 0.168, p = 0.015) improved significantly for women using 10 mg rapamycin. Self-reported emotional well-being (ηp2 = 0.108, p = 0.023) and general health (ηp2 = 0.166, p = 0.004) also improved for those using 5 mg rapamycin. No other significant effects were observed.
Conclusions: Low-dose, intermittent rapamycin administration over 48 weeks is relatively safe in healthy, normative-aging adults, and was associated with significant improvements in lean tissue mass and pain in women. Future work will evaluate benefits of a broader range of rapamycin doses on healthspan metrics for longevity, and will aim to more comprehensively establish efficacy.
Sign up for free Altmetric alerts about this article -
https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.206235
Subscribe for free publication alerts from Aging - https://www.aging-us.com/subscribe-to-toc-alerts
Keywords - aging, rapamycin, geroscience, longevity, healthspan
To learn more about the journal, please visit our website at https://www.Aging-US.com and connect with us on social media at:
Facebook - https://www.facebook.com/AgingUS/
X - https://twitter.com/AgingJrnl
Instagram - https://www.instagram.com/agingjrnl/
YouTube - https://www.youtube.com/@AgingJournal
LinkedIn - https://www.linkedin.com/company/aging/
Bluesky - https://bsky.app/profile/aging-us.bsky.social
Pinterest - https://www.pinterest.com/AgingUS/
Spotify - https://open.spotify.com/show/1X4HQQgegjReaf6Mozn6Mc
MEDIA@IMPACTJOURNALS.COM