Exploring the Link Between LSD and Anxiety
Description
STORY AT-A-GLANCE
LSD shows promise for anxiety treatment. A Phase 2b clinical trial found that 100 micrograms of MM120 (pharmaceutical LSD) reduced anxiety significantly with lasting effects
Study results were encouraging. Researchers noted that 65% of participants experienced clinical response at optimal dosing, with 47.5% achieving remission that sustained through 12 weeks of follow-up
Safety profile was manageable. Most side effects occurred during dosing sessions and resolved quickly, with visual changes and nausea being most common under medical supervision
Note that LSD remains illegal. It is currently classified as Schedule I controlled substance, accessible only through approved clinical trials, with results of Phase 3 trials expected by 2026
In the meantime, natural anxiety alternatives exist. Breathing techniques like nasal breathing, horizontal breathing patterns, and controlled breathing practices can help manage anxiety symptoms without medication

In a recent blog post by bioenergetics expert Georgi Dinkov, he highlighted the link between LSD and anxiety. Short for lysergic acid diethylamide, this drug has been banned for decades by the government for being highly “addictive,” as well as triggering psychosis among its users.1
However, as Dinkov pointed out, LSD is scaring Big Pharma because it is a potent antianxiety drug when taken under medical supervision. Moreover, the effects were long-lasting.2
This means that if LSD were to be released to the public under reasonable regulations, Big Pharma will suffer huge losses. After all, why would anyone buy their harmful and addictive antianxiety medication when they can just take a small dose? That said, here’s an analysis of the featured study.
Does LSD Help Anxiety? Here’s What Researchers Found
In a paper published in JAMA,3 researchers funded by biotechnology company MindMed published their findings of a multicenter, double-blind, placebo-controlled, Phase 2b study. For context, clinical trials are generally divided in three phases — 1, 2, and 3.4 This means at the time of publishing, the research has already expanded to medium-sized testing and analysis.
Context of the study — In 2020, MindMed published the findings of their Phase 1 trials of MM120,5 which is an “oral pharmaceutical formulation of LSD.”6 Upon positive results from that experiment, the U.S. Food and Drug Administration (FDA) granted permission to proceed to Phase 2 trials in 2024.7
Continuing the research — Now, the Phase 2b trials published in JAMA took place across 22 outpatient psychiatric research sites in the United States and involved 198 participants between the ages of 18 and 74. Every participant had a confirmed diagnosis of moderate-to-severe GAD, defined by a score of 20 or higher on the Hamilton Anxiety Rating Scale (HAM-A), a widely accepted clinical measure of anxiety severity.
Conducting Phase 2b trials — Participants were randomly assigned to one of five groups — placebo, 25 micrograms (μg), 50 μg, 100 μg, or 200 μg of MM120. The trial’s main measure of success was whether anxiety scores on the HAM-A dropped meaningfully after four weeks. A reduction of 2.5 points on the HAM-A is considered clinically important.
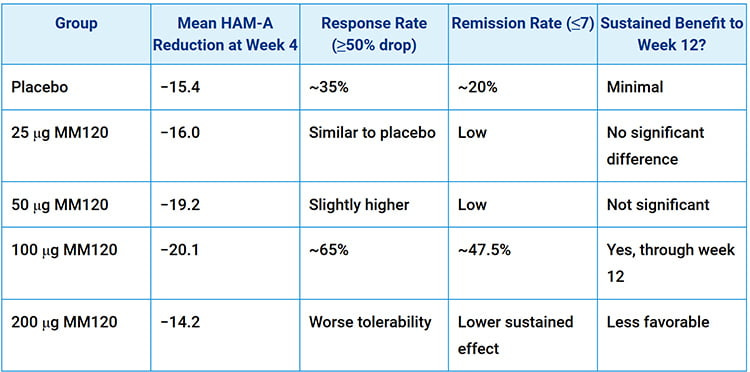
Findings of the trials — The standout result was that 100 micrograms worked best. At this dose, about 65% of participants experienced a clinical response, which means their anxiety scores fell by at least half. Even more compelling, 47.5% of these individuals reached remission, with anxiety levels so low they were considered minimal.
Following Up on the Participants — What Changed by Week 4 vs. Week 12
Taken altogether, the results were striking in how much MM120 impacted HAM-A scores. Below is a simplified table showing the effects at different doses:
The 100 μg dose stood out — Again, it had the best combination of strong anxiety reduction and sustained effects with manageable side effects.
Effects were better as time went on — By the four-week mark, the benefits of MM120 were already strong. The mean reduction in HAM-A scores at the 100 μg dose was −20.1 points, compared to −15.4 in the placebo group. While placebo effects were notable, the improvements at 100 μg and 200 μg went far beyond what would be expected from placebo alone.
What makes this trial stand out is th