Steve Quake and Charlotte Bunne: The Holy Grail of Biology
Description
“Eventually, my dream would be to simulate a virtual cell.”—Demis Hassabis
The aspiration to build the virtual cell is considered to be equivalent to a moonshot for digital biology. Recently, 42 leading life scientists published a paper in Cell on why this is so vital, and how it may ultimately be accomplished. This conversation is with 2 of the authors, Charlotte Bunne, now at EPFL and Steve Quake, a Professor at Stanford University, who heads up science at the Chan-Zuckerberg Initiative
The audio (above) is available on iTunes and Spotify. The full video is linked here, at the top, and also can be found on YouTube.
TRANSCRIPT WITH LINKS TO AUDIO
Eric Topol (00:06 ):
Hello, it's Eric Topol with Ground Truths and we've got a really hot topic today, the virtual cell. And what I think is extraordinarily important futuristic paper that recently appeared in the journal Cell and the first author, Charlotte Bunne from EPFL, previously at Stanford’s Computer Science. And Steve Quake, a young friend of mine for many years who heads up the Chan Zuckerberg Initiative (CZI) as well as a professor at Stanford. So welcome, Charlotte and Steve.
Steve Quake (00:42 ):
Thanks, Eric. It's great to be here.
Charlotte Bunne:
Thanks for having me.
Eric Topol (00:45 ):
Yeah. So you wrote this article that Charlotte, the first author, and Steve, one of the senior authors, appeared in Cell in December and it just grabbed me, “How to build the virtual cell with artificial intelligence: Priorities and opportunities.” It's the holy grail of biology. We're in this era of digital biology and as you point out in the paper, it's a convergence of what's happening in AI, which is just moving at a velocity that's just so extraordinary and what's happening in biology. So maybe we can start off by, you had some 42 authors that I assume they congregated for a conference or something or how did you get 42 people to agree to the words in this paper?
Steve Quake (01:33 ):
We did. We had a meeting at CZI to bring community members together from many different parts of the community, from computer science to bioinformatics, AI experts, biologists who don't trust any of this. We wanted to have some real contrarians in the mix as well and have them have a conversation together about is there an opportunity here? What's the shape of it? What's realistic to expect? And that was sort of the genesis of the article.
Eric Topol (02:02 ):
And Charlotte, how did you get to be drafting the paper?
Charlotte Bunne (02:09 ):
So I did my postdoc with Aviv Regev at Genentech and Jure Leskovec at CZI and Jure was part of the residency program of CZI. And so, this is how we got involved and you had also prior work with Steve on the universal cell embedding. So this is how everything got started.
Eric Topol (02:29 ):
And it's actually amazing because it's a who's who of people who work in life science, AI and digital biology and omics. I mean it's pretty darn impressive. So I thought I'd start off with a quote in the article because it kind of tells a story of where this could go. So the quote was in the paper, “AIVC (artificial intelligence virtual cell) has the potential to revolutionize the scientific process, leading to future breakthroughs in biomedical research, personalized medicine, drug discovery, cell engineering, and programmable biology.” That's a pretty big statement. So maybe we can just kind of toss that around a bit and maybe give it a little more thoughts and color as to what you were positing there.
Steve Quake (03:19 ):
Yeah, Charlotte, you want me to take the first shot at that? Okay. So Eric, it is a bold claim and we have a really bold ambition here. We view that over the course of a decade, AI is going to provide the ability to make a transformative computational tool for biology. Right now, cell biology is 90% experimental and 10% computational, roughly speaking. And you've got to do just all kinds of tedious, expensive, challenging lab work to get to the answer. And I don't think AI is going to replace that, but it can invert the ratio. So within 10 years I think we can get to biology being 90% computational and 10% experimental. And the goal of the virtual cell is to build a tool that'll do that.
Eric Topol (04:09 ):
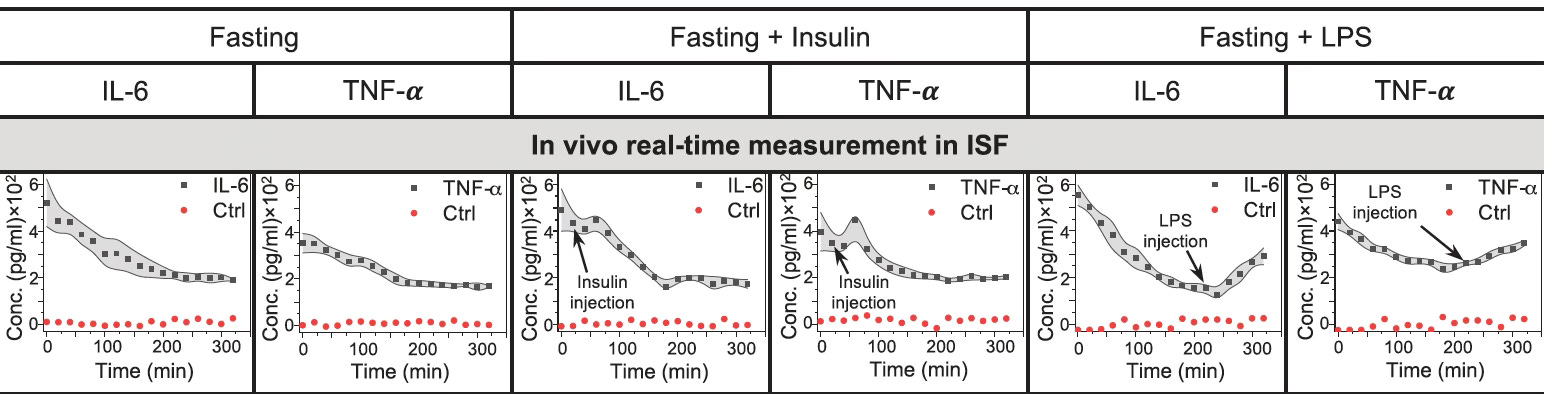
And I think a lot of people may not understand why it is considered the holy grail because it is the fundamental unit of life and it's incredibly complex. It's not just all the things happening in the cell with atoms and molecules and organelles and everything inside, but then there's also the interactions the cell to other cells in the outside tissue and world. So I mean it's really quite extraordinary challenge that you've taken on here. And I guess there's some debate, do we have the right foundation? We're going to get into foundation models in a second. A good friend of mine and part of this whole I think process that you got together, Eran Segal from Israel, he said, “We're at this tipping point…All the stars are aligned, and we have all the different components: the data, the compute, the modeling.” And in the paper you describe how we have over the last couple of decades have so many different data sets that are rich that are global initiatives. But then there's also questions. Do we really have the data? I think Bo Wang especially asked about that. Maybe Charlotte, what are your thoughts about data deficiency? There's a lot of data, but do you really have what we need before we bring them all together for this kind of single model that will get us some to the virtual cell?
Charlotte Bunne (05:41 ):
So I think, I mean one core idea of building this AIVC is that we basically can leverage all experimental data that is overall collected. So this also goes back to the point Steve just made. So meaning that we basically can integrate across many different studies data because we have AI algorithms or the architectures that power such an AIVC are able to integrate basically data sets on many different scales. So we are going a bit away from this dogma. I'm designing one algorithm from one dataset to this idea of I have an architecture that can take in multiple dataset on multiple scales. So this will help us a bit in being somewhat efficient with the type of experiments that we need to make and the type of experiments we need to conduct. And again, what Steve just said, ultimately, we can very much steer which data sets we need to collect.
Charlotte Bunne (06:34 ):
Currently, of course we don't have all the data that is sufficient. I mean in particular, I think most of the tissues we have, they are healthy tissues. We don't have all the disease phenotypes that we would like to measure, having patient data is always a very tricky case. We have mostly non-interventional data, meaning we have very limited understanding of somehow the effect of different perturbations. Perturbations that happen on many different scales in many different environments. So we need to collect a lot here. I think the overall journey that we are going with is that we take the data that we have, we make clever decisions on the data that we will collect in the future, and we have this also self-