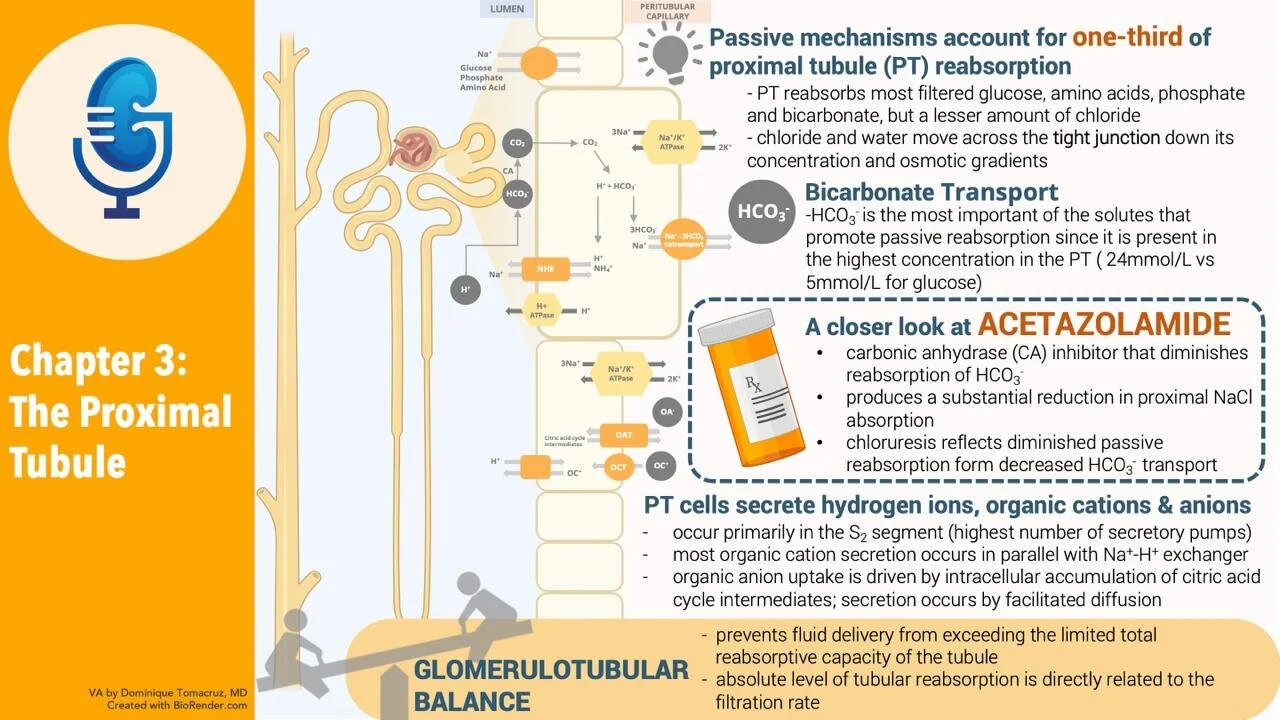
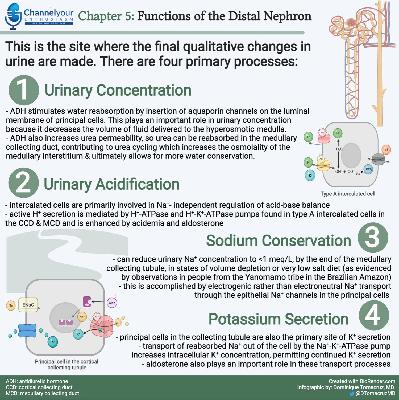
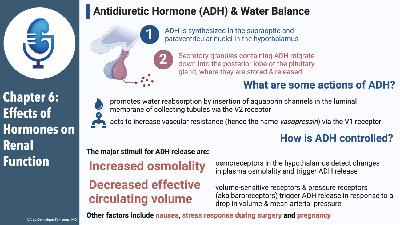
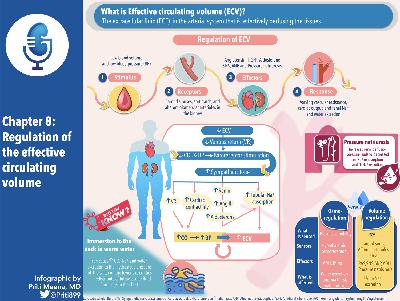
Chapter Eleven, part 2: Regulation of Acid-Base Balance
Description
References
We considered the complexity of the machinery to excrete ammonium in the context of research on dietary protein and how high protein intake may increase glomerular pressure and contribute to progressive renal disease (many refer to this as the “Brenner hypothesis”). Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease
A trial that studied low protein and progression of CKD The Effects of Dietary Protein Restriction and Blood-Pressure Control on the Progression of Chronic Renal Disease
(and famously provided data for the MDRD eGFR equation A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group
We wondered about dietary recommendations in CKD. of note, this is best done in the DKD guidelines from KDIGO Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment.
Joel mentioned this study on red meat and risk of ESKD. Red Meat Intake and Risk of ESRD
We referenced the notion of a plant-based diet. This is an excellent review by Deborah Clegg and Kathleen Hill Gallant. Plant-Based Diets in CKD : Clinical Journal of the American Society of Nephrology
Here’s the review that Josh mentioned on how the kidney appears to sense pH Molecular mechanisms of acid-base sensing by the kidney
Remarkably, Dr. Dale Dubin put a prize in his ECG book Free Car Prize Hidden in Textbook Read the fine print: Student wins T-bird
A review of the role of the kidney in DKA: Diabetic ketoacidosis: Role of the kidney in the acid-base homeostasis re-evaluated
Josh mentioned the effects of infusing large amounts of bicarbonate The effect of prolonged administration of large doses of sodium bicarbonate in man and this study on the respiratory response to a bicarbonate infusion: The Acute Effects In Man Of A Rapid Intravenous Infusion Of Hypertonic Sodium Bicarbonate Solution. Ii. Changes In Respiration And Output Of Carbon Dioxide
This is the study of acute respiratory alkalosis in dogs: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC293311/?page=1
And this is the study of medical students who went to the High Alpine Research Station on the Jungfraujoch in the Swiss Alps https://www.nejm.org/doi/full/10.1056/nejm199105163242003
Self explanatory! A group favorite! It Is Chloride Depletion Alkalosis, Not Contraction Alkalosis
A review of pendrin’s role in volume homeostasis: The role of pendrin in blood pressure regulation | American Journal of Physiology-Renal Physiology
Infusion of bicarbonate may lead to a decrease in respiratory stimulation but the shift of bicarbonate to the CSF may lag. Check out this review Neural Control of Breathing and CO2 Homeostasis and this classic paper Spinal-Fluid pH and Neurologic Symptoms in Systemic Acidosis.
Outline
Outline: Chapter 11
- Regulation of Acid-Base Balance
- Introduction
- Bicarb plus a proton in equilibrium with CO2 and water
- Can be rearranged to HH
- Importance of regulating pCO2 and HCO3 outside of this equation
- Metabolism of carbs and fats results in the production of 15,000 mmol of CO2 per day
- Metabolism of protein and other “substances” generates non-carbonic acids and bases
- Mostly from sulfur containing methionine and cysteine
- And cationic arginine and lysine
- Hydrolysis of dietary phosphate that exists and H2PO4–
- Source of base/alkali
- Metabolism of an ionic amino acids
- Glutamate and asparatate
- Organic anions going through gluconeogenesis
- Glutamate, Citrate and lactate
- Net effect on a normal western diet 50-100 mEq of H+ per day
- Homeostatic response to these acid-base loads has three stages:
- Chemical buffering
- Changes in ventilation
- Changes in H+ excretion
- Example of H2SO4 from oxidation of sulfur containing AA
- Drop in bicarb will stimulate renal acid secretion
- Nice table of normal cid-base values, arterial and venous
- Great 6 bullet points of acid-base on page 328
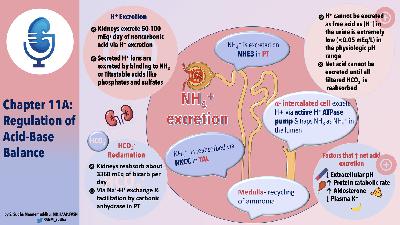
- Kidneys must excrete 50-100 of non-carbonic acid daily
- This occurs by H secretion, but mechanisms change by area of nephron
- Not excreted as free H+ due to minimal urine pH being equivalent to 0.05 mmol/L
- No H+ can be excreted until virtually all of th filtered bicarb is reabsorbed
- Secreted H+ must bind buffers (phosphate, NH3, cr)
- PH is main stimulus for H secretion, though K, aldo and volume can affect this.
- Renal Hydrogen excretion
- Critical to understand that loss of bicarb is like addition of hydrogen to the body
- So all bicarb must be reabsorbed before dietary H load can be secreted
- GFR of 125 and bicarb of 24 results in 4300 mEq of bicarb to be reabsorbed daily
- Reabsorption of bicarb and secretion of H involve H secretion from tubular cells into the lumen.
- Thee initial points need to be emphasized
<p class="" style="white-space: pre-wr